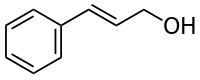
Cinnamyl alcohol
 | |
| Names | |
|---|---|
| IUPAC name
(2E)-3-phenylprop-2-en-1-ol | |
| Other names
Cinnamyl alcohol Cinnamic alcohol Styryl carbinol Phenylallyl alcohol | |
| Identifiers | |
| 104-54-1 | |
| ChEBI | CHEBI:33227 |
| ChEMBL | ChEMBL324794 |
| ChemSpider | 21105870 |
| Jmol interactive 3D | Image |
| KEGG | C02394 |
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.17 g/mol |
| Density | 1.0397 g/cm3 at 35 °C |
| Melting point | 33 °C |
| Boiling point | 250 °C |
| Hazards | |
| Safety data sheet | External MSDS |
| Related compounds | |
| Related compounds |
Cinnamic acid; Cinnamaldehyde |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Cinnamyl alcohol is an organic compound that is found in esterified form in storax, Balsam of Peru, and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the hydrolysis of storax.
Cinnamyl alcohol has a distinctive odour described as "sweet, balsam, hyacinth, spicy, green, powdery, cinnamic" and is used in perfumery[1] and as a deodorant.
Safety
Cinnamyl alcohol has been found to have a sensitising effect on some people[2][3] and as a result is the subject of a Restricted Standard issued by IFRA (International Fragrance Association).
Glycosides
Rosarin and rosavin are cinnamyl alcohol glycosides isolated from Rhodiola rosea.
References
- ↑ "cinnamyl alcohol 104-54-1". thegoodscentscompany.com. Retrieved 26 July 2015.
- ↑ http://www.rifm.org/doc/Food%20&%20Chem%20Tox%20RIFM%20Spec%20Suppl%20122007.pdf
- ↑ Survey and health assessment of chemical substances in massage oils
- Merck Index, 11th Edition, 2305.
|
This article is issued from Wikipedia - version of the Monday, November 16, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.