Chytridiomycosis

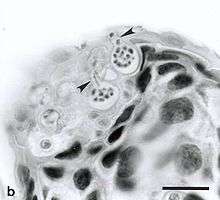
Chytridiomycosis is an infectious disease in amphibians, caused by the chytrid Batrachochytrium dendrobatidis, a nonhyphal zoosporic fungus. Chytridiomycosis has been linked to dramatic population declines or even extinctions of amphibian species in western North America, Central America, South America, eastern Australia, and Dominica and Montserrat in the Caribbean. Much of the New World is also at risk of the disease arriving within the coming years.[1] The fungus is capable of causing sporadic deaths in some amphibian populations and 100% mortality in others. No effective measure is known for control of the disease in wild populations. Various clinical signs are seen by individuals affected by the disease. A number of options are possible for controlling this disease-causing fungus, though none has proved to be feasible on a large scale. The disease has been proposed as a contributing factor to a global decline in amphibian populations that apparently has affected about 30% of the amphibian species of the world.[2]
History
The disease in its epizootic form was first discovered in 1993 in dead and dying frogs in Queensland, Australia. It had been present in the country since at least 1978 and is widespread across Australia. It is also found in Africa, the Americas, Europe, New Zealand, and Oceania. In Australia, Panama, and New Zealand, the fungus seemed to have suddenly ‘appeared’ and expanded its range at the same time frog numbers declined. However, it may simply be that the fungus occurs naturally and was only identified recently because it has become more virulent or more prevalent in the environment, or because host populations have become less resistant to the disease. The fungus has been detected in four areas of Australia—the east coast, Adelaide, south-west Western Australia and the Kimberley—and is probably present elsewhere.[3]
The oldest documented occurrence of Batrachochytrium is from a Japanese giant salamander collected in 1902, although this strain of the fungus belongs to an endemic lineage that has not been implicated in any mass mortality events.[4] The next known instance of a Bd-infected amphibian specimen of an African clawed frog (Xenopus laevis) collected in 1938, and this species also appears to be essentially unaffected by the disease, making it a suitable vector.[5] The first well-documented method of human pregnancy testing involved this species, and as a result, large-scale international trade in living African clawed frogs began more than 60 years ago.[5] If Batrachochytrium originated in Africa, it has been theorized that the African clawed frog was the vector of the initial spread out of the continent.[5] The earliest documented case of the disease chytridiomycosis was an American bullfrog (Rana catesbeiana) collected in 1978.[5] It is still not clear if it is a new emergent pathogen or if it is an old pathogen with recently increased virulence.
Range
The geographic range of chytridiomycosis is difficult to ascertain. If it occurs, the disease is only present where the fungus B. dendrobatidis is present. However, the disease is not always present where the fungus is. Reasons for amphibian declines are often termed ‘enigmatic’ because the cause is unknown. Why some areas are affected by the fungus while others are not is not fully understood. Oscillating factors such as climate, habitat suitability, and population density may be factors which cause the fungus to infect amphibians of a given area. Therefore, when considering the geographic range of chytridiomycosis, the range of B. dendrobatidis occurrence must be considered.[6]
The geographic range of B. dendrobatidis has recently been mapped, and spans much of the world. B. dendrobatidis has been detected in 56 of 82 countries, and in 516 of 1240 (42%) species using a data set of more than 36,000 individuals. It is widely distributed in the Americas, and detected sporadically in Africa, Asia, and Europe.[1] Asia, for example, has only 2.35% prevalence.[7]
The range suitable for B. dendrobatidis in the New World is vast. Regions with its highest suitability include habitats that contain the world’s most diverse amphibian fauna. Areas at risk are the Sierra Madre Pine Oak Occidental Forest, the Sonoran and Sinaloan dry forest, the Veracruz moist forest, Central America east from the Isthmus of Tehuantepec, the Caribbean Islands, the temperate forest in Chile and western Argentina south of 30°S, the Andes above 1000 m above sea level in Venezuela, Colombia, and Ecuador, eastern slopes of the Andes in Peru and Bolivia, the Brazilian Atlantic forest, Uruguay, Paraguay, and northeastern Argentina, as well as the southwestern and Madeira-Tapaj Amazonian rainforests.[8]
Currently, the effects of chytridiomycosis are seen most readily in Central America, eastern Australia, South America, and western North America.[1]
Causative agents
Chytridiomycosis caused by the fungus B. dendrobatidis predominantly affects the outermost layers of skin containing keratin.[6] When most species reach a B. dendrobatidis threshold of 10,000 zoospores, they are not able to breathe, hydrate, osmoregulate, or thermoregulate correctly. This is proven by blood samples that show a lack of certain electrolytes, such as sodium, magnesium, and potassium. B. dendrobatidis is currently known to have two life stages. The first is the asexual zoosporangial stage.[9] When a host first contracts the disease, spores penetrate the skin and attach themselves using microtubule roots.[10] The second stage takes place when the initial asexual zoosporangia produce motile zoospores.[9] To disperse and infect epidermal cells, a wet surface is needed.[9] A second species of Batrachochytrium, B. salamandrivorans, was discovered in 2013 and is known to cause chytridiomycosis in salamanders.[11]
Disease transmission and progression
B. dendrobatidis, a waterborne pathogen, disperses zoospores into the environment.[12] The zoospores use flagella for locomotion through water systems until they reach a new host and enter cutaneously.[10] The B. dendrobatidis’ lifecycle continues until new zoospores are produced from the zoosporangium and exit to the environment or reinfect the same host.[10] Once the host is infected with B. dendrobatidis, it can potentially develop chytridiomycosis, but not all infected hosts develop it.[10] Other forms of transmission are currently unknown; however, chytridiomycosis is postulated to be transmitted through direct contact of hosts or through an intermediate host.[10]
Much of how B. dendrobatidis is successfully transmitted from one host to the next is largely unknown.[13] Once released into the aquatic environment, zoospores travel less than 2 cm within 24 hours before they encyst.[14] The limited range of B. dendrobatidis zoospores suggest some unknown mechanism exists by which they transmit from one host to the next.[14] Abiotic factors such as temperature, pH level, and nutrient levels affect the success of B. dendrobatidis zoospores.[14] The fungus zoospores can survive within a temperature range of 4–25 °C and a pH range of 6–7.[14]
Chytridiomycosis is believed to follow this course: zoospores first encounter amphibian skin and quickly give rise to sporangia, which produce new zoospores.[15] The disease then progresses as these new zoospores reinfect the host. Morphological changes in amphibians infected with the fungus include a reddening of the ventral skin, convulsions with extension of hind limbs, accumulations of sloughed skin over the body, sloughing of the superficial epidermis of the feet and other areas, slight roughening of the surface with minute skin tags, and occasional small ulcers or hemorrhage. Behavioral changes can include lethargy, a failure to seek shelter, a failure to flee, a loss of righting reflex, and abnormal posture (e.g. sitting with the hind legs away from the body).[16]
Clinical signs
Amphibians infected with B. dendrobatidis have been known to show many different clinical signs. Perhaps the earliest sign of infection is anorexia, occurring as quickly as 8 days after being exposed .[13] Individuals infected are also commonly found in a lethargic state, characterized by slow movements, and refuse to move when stimulated. Excessive shedding of skin is seen in most frog species affected by B. dendrobatidis.[6] These pieces of shed skin are described as opaque, gray-white, and tan.[6] Some of these patches of skin are also found adhered to the skin of the amphibians.[6] These signs of infection are often seen 12–15 days following exposure.[13] The most typical symptom of chytridomycosis is thickening of skin, which promptly leads to the death of the infected individuals because those individuals cannot take in the proper nutrients, release toxins, or, in some cases, breathe.[6] Other common signs are reddening of the skin, convulsions, and a loss of righting reflex .[13] In tadpoles B. dendrobatidis affects the mouthparts, where keratin is present, leading to abnormal feeding behaviors or discoloration of the mouth.[6]
Research
The amphibian chytrid fungus appears to grow best between 17 and 25 °C,[14] and exposure of infected frogs to high temperatures can cure the frogs.[17] In nature, the more time individual frogs were found at temperatures above 25 °C, the less likely they were to be infected by the amphibian chytrid.[18] This may explain why chytridiomycosis-induced amphibian declines have occurred primarily at higher elevations and during cooler months.[19] Naturally produced cutaneous peptides can inhibit the growth of B. dendrobatidis when the infected amphibians are around temperatures near 10 °C (50 °F), allowing species like the northern leopard frog (Rana pipiens) to clear the infection in about 15% of cases.[20]
Although many declines have been credited to the fungus B. dendrobatidis, some species resist the infection and some populations can survive with a low level of persistence of the disease.[21] In addition, some species that seem to resist the infection may actually harbor a nonpathogenic form of B. dendrobatidis.
Some researchers contend the focus on chytridiomycosis has made amphibian conservation efforts dangerously myopic. A review of the data in the IUCN Red List found the threat of the disease was assumed in most cases, but no evidence shows, in fact, it is a threat.[22] Conservation efforts in New Zealand continue to be focused on curing the critically endangered native Archey's frog, Leiopelma archeyi, of chytridiomycosis, though research has shown clearly that they are immune from infection by B. dendrobatidis and are dying in the wild of other still-to-be identified diseases.[23] In Guatemala, several thousand tadpoles perished from an unidentified pathogen distinct from B. dendrobatidis.[24]
Treatment options
Treatment options for chytridiomycosis include antifungal drugs and heat-induced therapy.[25] The antifungal drug itraconazole is the most popular form of treatment.[9][25][26][27] Individuals infected with B. dendrobatidis are bathed in intraconazole solutions, and within a few weeks, previously infected individuals test negative for B. dendrobatidis using PCR assays.[9][26][27] Heat therapy is also used to neutralize B. dendrobatidis in infected individuals.[25][28] Temperature-controlled laboratory experiments are used to increase the temperature of an individual past the optimal temperature range of B. dendrobatidis.[28] Experiments, where the temperature is increased beyond the upper bound of the B. dendrobatidis optimal range of 25 to 30 °C, show its presence will dissipate within a few weeks and individuals infected return to normal.[28] Formalin/malachite green has also been used to successfully treat individuals infected with chytridiomycosis.[9] An Archey's frog was successfully cured of chytridiomycosis by applying chloramphenicol topically.[29] However, the potential risks of using antifungal drugs on individuals are high.[25]
Immunity hypothesis
Due to the fungus' immense impact on amphibian populations, considerable research has been undertaken to devise methods to combat its proliferation in the wild. Among the most promising is the revelation that amphibians in colonies that survive the passage of the chytrid epidemic tend to carry higher levels of the bacterium Janthinobacterium lividum.[30] This bacterium produces antifungal compounds, such as indole-3-carboxaldehyde and violacein, that inhibit the growth of B. dendrobatidis even at low concentrations.[31] Similarly, the bacterium Lysobacter gummosus found on the red-backed salamander (Plethodon cinereus), produces the compound 2,4-diacetylphloroglucinol that is inhibitory to the growth of B. dendrobatidis.[32]
Understanding the interactions of microbial communities present on amphibians’ skin with fungal species in the environment can reveal why certain amphibians, such as the frog Rana muscosa, are susceptible to the fatal effects of B. dendrobatidis and why others, such as the salamander Hemidactylium scutatum, are able to coexist with the fungus. As mentioned before, the antifungal bacterial species Janthinobacterium lividum, found on several amphibian species, has been shown to prevent the effects of the pathogen even when added to another amphibian that lacks the bacteria (B. dendrobatidis-susceptible amphibian species).[33] Interactions between cutaneous microbiota and B. dendrobatidis can be altered to favor the resistance of the disease, as seen in past studies concerning the addition of the violacein-producing bacteria J. lividum to amphibians that lacked sufficient violacein, allowing them to inhibit infection.[34][35] Although the exact concentration of violacein (antifungal metabolite produced by J. lividum) needed to inhibit the effects of B. dendrobatidis is not fully confirmed, violacein concentration can determine whether or not an amphibian will experience morbidity (or mortality) caused by B. dendrobatidis. The frog Rana muscosa, for example, has been found to have very low concentrations of violacein on its skin, yet the concentration is so small, it is unable to facilitate increased survivability of the frog; furthermore, J. lividum has not been found to be present on the skin of R. muscosa.[33][36] This implies that the antifungal bacteria J. lividum (native to other amphibians' skin, such as Hemidactylium scutatum) is able to produce a sufficient amount of violacein to prevent infection by B. dendrobatidis and allow coexistence with the potentially deadly fungus.
One study has postulated that the water flea Daphnia magna eats the spores of the fungus.[37]
Interactions with pesticides
The hypothesis that pesticide use has contributed to declining amphibian populations has been suggested several times in the literature.[38][39][40] Interactions between pesticides and chytridiomycosis were examined in 2007, and sublethal exposure to the pesticide carbaryl (a cholinesterase inhibitor) was shown to increase susceptibility of foothill yellow-legged frogs (Rana boylii) to chytridiomycosis. In particular, the skin peptide defenses were significantly reduced after exposure to carbaryl, suggesting pesticides may inhibit this innate immune defence, and increase susceptibility to disease.[41]
See also
- Emerging infectious diseases
- White nose syndrome
- Gastric-brooding frog
- Golden toad
- Guajira stubfoot toad
- Rabb's Fringe-limbed Treefrog
- Holocene extinction
References
- 1 2 3 Olson, Deanna H.; Aanensen, David M.; Ronnenberg, Kathryn L.; Powell, Christopher I.; Walker, Susan F.; Bielby, Jon; Garner, Trenton W. J.; Weaver, George; Fisher, Matthew C.; Stajich, Jason E. (2013). Stajich, Jason E, ed. "Mapping the Global Emergence of Batrachochytrium dendrobatidis, the Amphibian Chytrid Fungus". PLoS ONE 8 (2): e56802. doi:10.1371/journal.pone.0056802. PMC 3584086. PMID 23463502.

- ↑ Stuart S. N., Chanson J. S.; et al. (2004). "Status and trends of amphibian declines and extinctions worldwide". Science 306 (5702): 1783–1786. doi:10.1126/science.1103538. PMID 15486254.
- ↑ "Chytridiomycosis (Amphibian Chytrid Fungus Disease)" (PDF). Australian Government Department of Sustainability, Environment, Water, Population and Communities. Retrieved 14 October 2013.
- ↑ Goka, Koichi; Yokoyama, Jun; Une, Yumi; Kuroki, Toshiro; Suzuki, Kazutaka; Nakahara, Miri; Kobayashi, Arei; Inaba, Shigeki; Mizutani, Tomoo; Hyatt, Alex D. (2009). "Amphibian chytridiomycosis in Japan: distribution,haplotypes and possible route of entry into Japan". Molecular Ecology 18: 4757–4774. doi:10.1111/j.1365-294x.2009.04384.x. Retrieved 6 February 2016.
- 1 2 3 4 Weldon; du Preez; Hyatt; Muller; and Speare (2004). Origin of the Amphibian Chytrid Fungus. Emerging Infectious Disease 10(12).
- 1 2 3 4 5 6 7 Whittaker, Kellie, and Vance Vredenburg. "An Overview of Chytridiomycosis." AMPHIBIAWEB. University of California, 17 May 2011. Web. 23 Oct 2013. <http://www.amphibiaweb.org/chytrid/chytridiomycosis.html.
- ↑ Swei, A.; Rowley, J.J. L.; Rödder, D.; Diesmos, M. L. L.; Diesmos, A. C.; Briggs, C. J.; Brown, R.; et al. (2011). Arlettaz, Raphaël, ed. "Is Chytridiomycosis an Emerging Infectious Disease in Asia?". PLoS ONE 6 (8): e23179. doi:10.1371/journal.pone.0023179. PMC 3156717. PMID 21887238.

- ↑ Ron, Santiago R. (June 2005). "Predicting the Distribution of the Amphibian Pathogen Batrachochytrium dendrobatidis in the New World". Biotropica 37 (2): 209–221. doi:10.1111/j.1744-7429.2005.00028.x.
- 1 2 3 4 5 6 Parker, J. M., I. Mikaelian, N. Hahn, and H. E. Diggs. 2002. Clinical diagnosis and treatment of epidermal chytridiomycosis in African clawed frogs (Xenopus tropicalis). American Association for Laboratory Animal Science 52:265–268.
- 1 2 3 4 5 Longcore J. E., Pessier A. P., Nichols D. K. (1999). "Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians". Mycologia 91: 219–227. doi:10.2307/3761366.
- ↑ Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M. C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; Pasmans, F. (2013). "Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians". Proceedings of the National Academy of Sciences of the United States of America 110 (38): 15325–15329. doi:10.1073/pnas.1307356110. PMC 3780879. PMID 24003137.
- ↑ Morgan J. A. T., Vredenburg V. T., Rachowicz L. J., Knapp R. A., Stice M. J., Tunstall T., Bingham R. E., Parker J. M., Longcore J. E.; et al. (2007). "Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis". Proceedings of the National Academy of Sciences of the United States of America 104: 13845–13850. doi:10.1073/pnas.0701838104.
- 1 2 3 4 Nichols, D. K., E. W. Lamirande, A. P. Pessier, and J. E. Longcore. 2001. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. Journal of Wildlife Diseases 37:1–11.
- 1 2 3 4 5 Piotrowski J. S., Annis S. L., Longcore J. E. (2004). "Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians". Mycologia 96: 9–15. doi:10.2307/3761981.
- ↑ Berger, L., Hyatt, A.D., Speare, R. & Longcore, J.E. Dis. Aquat. Org. 68, 51–63 (2005).
- ↑ Padgett-Flohr, G.E. (2007). "Amphibian Chytridiomycosis: An Informational Brochure" (PDF). California Center for Amphibian Disease Control. Retrieved 14 October 2013.
- ↑ Woodhams, D. C., R. A. Alford, et al. (2003). "Emerging disease of amphibians cured by elevated body temperature." Diseases of aquatic organisms 55: 65–67.
- ↑ Rowley, J.J.L. & Alford, R.A. (2013) Hot bodies protect amphibians against chytrid infection in nature. Scientific Reports 3, 1515. doi:10.1038/srep01515
- ↑ Woodhams, D. C. & Alford, R. A. (2005) The ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conserv. Biol. 19, 1449–1459
- ↑ Voordouw, M. J., D. Adama, B. Houston, and P. Govindarajulu. "Prevalence of the Pathogenic Chytrid Fungus, Batrachochytrium Dendrobatidis, in an Endangered Population of Northern Leopard Frogs, Rana Pipiens." BMC Ecol. 6th ser. 10.1 (2010). Print.
- ↑ Retallick R. W. R., McCallum H.; et al. (2004). "Endemic Infection of the Amphibian Chytrid Fungus in a Frog Community Post-Decline". PLoS Biology 2 (11): e351. doi:10.1371/journal.pbio.0020351.

- ↑ Heard M, Smith KF, Ripp K (2011). "Examining the Evidence for Chytridiomycosis in Threatened Amphibian Species". PLoS ONE 6 (8): e23150. doi:10.1371/journal.pone.0023150.

- ↑ Waldman B (2011) Brief encounters with Archey's Frog. FrogLog 99:39–41.
- ↑ Di Rosa, Ines; Simoncelli, Francesca; Fagotti, Anna; Pascolini, Rita (2007). "Ecology: The proximate cause of frog declines?". Nature 447 (7144): E4–E5. doi:10.1038/nature05941. PMID 17538572.
- 1 2 3 4 Woodhams, D. C., C. C. Geiger, L. K. Reinert, L. A. Rollins-Smith, B. Lam, R. N. Harris, C. J. Briggs, V. T. Vredenburg, and J. Voyles. 2012. Treatment of amphibians infected with chytrid fungus: learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Diseases of Aquatic Organisms 98:11–25.
- 1 2 Une, Y., K. Matsui, K. Tamukai, and K. Goka. 2012. Eradication of the chytrid fungus Batrachochytrium dendrobatidis in the Japanese giant salamander Andrias japonicus. Diseases of Aquatic Organisms 98:243–247.
- 1 2 Jones, M. E. B., D. Paddock, L. Bender, J. L. Allen, M. D. Schrenzel, and A. P. Pessier. 2012. Treatment of chytridiomycosis with reduced-dose itraconazole. Diseases of Aquatic Organisms 99:243–249.
- 1 2 3 Chatfield, M. W. H. and C. L. Richards-Zawacki. 2011. Elevated temperature as a treatment for Barachochytrium dendrobatidis infection in captive frogs. Diseases of Aquatic Organisms 94:235–238.
- ↑ Bishop, PJ; Speare, R; Poulter, R; Butler, M; Speare, BJ; Hyatt, A; Olsen, V; Haigh, A (9 March 2009). "Elimination of the amphibian chytrid fungus Batrachochytrium dendrobatidis by Archey's frog Leiopelma archeyi" (PDF). Diseases of Aquatic Organisms 84 (1): 9–15. doi:10.3354/dao02028. PMID 19419002.
- ↑ (BBC News) Richard Black, " Bacteria could stop frog killer" Accessed 7 June 2008.
- ↑ Brucker RM, Harris RN, Schwantes CR, Gallaher TN, Flaherty DC, Lam BA, Minbiole KP (November 2008). "Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus". J. Chem. Ecol. 34 (11): 1422–9. doi:10.1007/s10886-008-9555-7. PMID 18949519.
- ↑ Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KP (January 2008). "The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus". J. Chem. Ecol. 34 (1): 39–43. doi:10.1007/s10886-007-9352-8. PMID 18058176.
- 1 2 Harris R., Brucker R., Minbiole K., Walke J., Becker M., Schwantes C.; et al. (2009). "Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus". ISME Journal 3 (7): 818–824. doi:10.1038/ismej.2009.27. PMID 19322245.
- ↑ Becker M., Brucker R., Schwantes C., Harris R., Minbiole K. (2009). "The bacterially-produced metabolite violacein is associated with survival in amphibians infected with a lethal disease". AEM 21: 1294–1302.
- ↑ Brucker R., Harris R., Schwantes C., Gallaher T., Flaherty D., Lam B.; et al. (2008). "Amphibian Chemical Defense: Antifungal Metabolites of the Microsymbiont Janthinobacterium lividum on the Salamander Plethodon cinereus". Springer 34 (11): 1422–1429. doi:10.1007/s10886-008-9555-7. PMID 18949519.
- ↑ Lam B., Walke J., Vredenburg V., Harris R. (2009). "Proportion of individuals with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa". Biological Conservation 143 (2): 529–531. doi:10.1016/j.biocon.2009.11.015.
- ↑ Buck, Julia; Truong, Lisa; Blaustein, Andrew (2011). "Predation by zooplankton on Batrachochytrium dendrobatidis: biological control of the deadly amphibian chytrid fungus?". Biodiversity and Conservation 20 (14): 3549–3553. doi:10.1007/s10531-011-0147-4.
- ↑ Stebbins, Robert C; Cohen, Nathan W. (1995). A Natural History of Amphibians. Princeton, N.J: Princeton University Press. ISBN 0-691-10251-1.
- ↑ Daividson C, Shaffer HB, Jennings MR (2001). "Declines of the California red-legged frog: climate, UV-B, habitat, and pesticides hypotheses". Ecological Applications 11 (2): 464–479. doi:10.1890/1051-0761(2001)011[0464:DOTCRL]2.0.CO;2.
- ↑ Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, Tsui M (April 2006). "Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact?". Environ. Health Perspect. 114 (Suppl 1): 40–50. doi:10.1289/ehp.8051. PMC 1874187. PMID 16818245.
- ↑ Davidson C, Benard MF, Shaffer HB, Parker JM, O'Leary C, Conlon JM, Rollins-Smith LA (March 2007). "Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs". Environ. Sci. Technol. 41 (5): 1771–6. doi:10.1021/es0611947. PMID 17396672.
External links
| Wikinews has related news: Frog-killing fungus spreads across Panama Canal towards South America |
- Article in National Geographic Magazine, April 2009
- Wildlife Trade and Global Disease Emergence
- Main preventative management strategies for the Chytrid fungus
- Amphibian chytridiomycosis at Amphibian Diseases Home Page
- 'Amphibian Ark' aims to save frogs from fungus
|