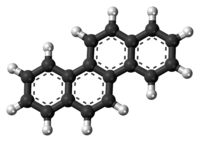
Chrysene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Chrysene | |
| Other names
Benzo[a]phenanthrene 1,2-Benzphenanthrene | |
| Identifiers | |
| 218-01-9 | |
| ChEBI | CHEBI:51687 |
| ChEMBL | ChEMBL85685 |
| ChemSpider | 8817 |
| EC Number | 205-923-4 |
| Jmol interactive 3D | Image |
| KEGG | C14222 |
| PubChem | 10457109 |
| RTECS number | GC0700000 |
| UNII | 084HCM49PT |
| |
| |
| Properties | |
| C18H12 | |
| Molar mass | 228.29 g·mol−1 |
| Appearance | Orthorhombic bipyramidal plates |
| Density | 1.274 g/cm3 |
| Melting point | 254 °C (489 °F; 527 K) |
| Boiling point | 448 °C (838 °F; 721 K) |
| Insoluble | |
| Solubility in ethanol | 1 g/1300 mL[2] |
| Related compounds | |
| Related PAHs |
Pyrene, Tetracene, Triphenylene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote, a chemical used to preserve wood.
The name "chrysene" originates from Greek Χρύσoς (chrysos), meaning "gold", and is due to the golden-yellow color of the crystals of the hydrocarbon, thought to be the proper color of the compound at the time of its isolation and characterization. However, high purity chrysene is colorless, the yellow hue being due to the traces of its yellow-orange isomer tetracene, which cannot be separated easily.
Chrysene is formed in small amounts during the burning or distillation of coal, crude oil, and plant material.
In February 2014, NASA announced a greatly upgraded database for tracking PAHs, including chrysene, in the universe.[3] According to scientists, more than 20% of the carbon in the universe may be associated with PAHs, possible starting materials for the formation of life. PAHs seem to have been formed shortly after the Big Bang, are widespread throughout the universe, and are associated with new stars and exoplanets.[4]
Use
Chrysene is used in the manufacture of some dyes.
Safety
As with other PAHs, chrysene is suspected to be a human carcinogen. It is known to cause cancer in laboratory animals.[5]
Derivatives
Derivatives of chrysene include tetrahydrochrysene and 2,8-dihydroxyhexahydrochrysene, which are estrogenic compounds.
See also
References
- ↑ Merck Index, 11th Edition, 2259.
- ↑ Merck Index, 14th edition
- ↑ NASA Ames PAH IR Spectroscopic Database
- ↑ Hoover, Rachel (February 21, 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". NASA. Retrieved February 22, 2014.
- ↑ TOXICOLOGICAL PROFILE FOR POLYCYCLIC AROMATIC HYDROCARBONS
| ||||||||||||||||||||||