Chidamide
 | |
| Systematic (IUPAC) name | |
|---|---|
|
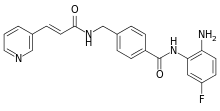
N-(2-Amino-5-fluorophenyl)-4-[[[1-oxo-3-(3-pyridinyl)-2-propen-1-yl]amino]methyl]-benzamide | |
| Clinical data | |
| Trade names | Epidaza |
| Identifiers | |
| CAS Number | 743420-02-2 |
| UNII |
87CIC980Y0 |
| Chemical data | |
| Formula | C22H19FN4O2 |
| Molar mass | 390.4 g/mol |
| |
Chidamide (Epidaza) is an HDAC inhibitor developed wholly in China that shows potential in treating pancreatic cancer.[1][2][3]
As of April 2015 it is only approved in China.[4]
Is NOT approved for the treatment of pancreatic cancer. Is approved by the Chinese FDA for relapsed or refractory peripheral T cell lymphoma.
References
- ↑ Qiao, Z (2013-04-26). "Chidamide, a novel histone deacetylase inhibitor, synergistically enhances gemcitabine cytotoxicity in pancreatic cancer cells.". Biochem Biophys Res Commun. 434 (1): 95–101. doi:10.1016/j.bbrc.2013.03.059. PMID 23541946.
- ↑ Guha, Malini (2015-04-01). "HDAC inhibitors still need a home run, despite recent approval". Nature Reviews Drug Discovery 14: 225–226. doi:10.1038/nrd4583.
- ↑ Wang, Shirley S. (2015-04-02). "A New Cancer Drug, Made in China". The Wall Street Journal. Retrieved 13 April 2015.
- ↑ "China's First Homegrown Pharma.". April 2015.
This article is issued from Wikipedia - version of the Sunday, January 10, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.