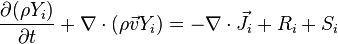Chemical reaction model
Chemical reaction models transform physical knowledge into a mathematical formulation so that that knowledge can be utilized in computational simulation of practical problems. The computers then provide the flexibility to visualize the phenomenon and their interactions to a wide range applications. Modeling of a chemical reaction involves solving conservation equations describing convection, diffusion, and reaction source for each component species.
Species transport equation
Ri is the net rate of production of species i by chemical reaction and Si is the rate of creation by addition from the dispersed phase and the user defined source. Ji is the diffusion flux of species i, which arises due to concentration gradients and differs in both laminar and turbulent flows. In turbulent flows, computational fluid dynamics also considers the effects of turbulent diffusivity. The net source of chemical species i due to reaction, Ri which appeared as the source term in the species transport equation is computed as the sum of the reaction sources over the NR reactions that the species participate in.
Reaction models
These reaction rates R can be calculated by following models
Laminar finite rate model
The laminar finite rate model computes the chemical source terms using the Arrhenius expressions and ignores turbulence fluctuations. This model provides with the exact solution for laminar flames but gives inaccurate solution for turbulent flames, in which turbulence highly affects the chemistry reaction rates, due to highly non-linear Arrhenius chemical kinetics. However this model may be accurate for combustion with small turbulence fluctuations example supersonic flames.
Eddy dissipation model
The eddy dissipation model, based on the work of Magnussen and Hjertager is a turbulent-chemistry reaction model. Most fuels are fast burning and the overall rate of reaction is controlled by turbulence mixing. In the non-premixed flames, turbulence slowly mixes the fuel and oxidizer into the reaction zones where they burn quickly. In premixed flames the turbulence slowly mixes cold reactants and hot products into the reaction zones where reaction occurs rapidly. In such cases the combustion is said to be mixing-limited, and the complex and often unknown chemical kinetics can be safely neglected. In this model, the chemical reaction is governed by large eddy mixing time scale. Combustion initiates whenever there is turbulence present in the flow. It does no need an ignition source to initiate the combustion. It is good for the non-premixed combustion but for the premixed flames the reactant will burn at the moment they enter in the computation model, which is incorrect. The reactant need some time to get to the ignition temperature to initiate the combustion. It is a shortcoming of this model.
Eddy dissipation concept
The eddy dissipation concept (EDC) model is an extension of the eddy dissipation model to include detailed chemical mechanism in turbulent flows. The EDC model attempts to incorporate the significance of fine structures in a turbulent reacting flows in which combustion is important. EDC has been proven efficient without the need for changing the constants for a great variety of premixed and diffusion controlled combustion problems, both where the chemical kinetics is faster than the overall fine structure mixing as well as in cases where the chemical kinetics has a dominating influence.
References
- Ansys Fluent Help, Chapter 7.
- Ansys Fluent Help, Chapter 8.
- Henk Kaarle Versteeg, Weeratunge Malalasekera,An Introduction to Computational Fluid Dynamics: The Finite Volume Method
- Bjørn F. Magnussen Norwegian University of Science and Technology Trondheim (Norway),Computational Industry Technologies AS (ComputIT),THE EDDY DISSIPATION CONCEPT A BRIDGE BETWEEN SCIENCE AND TECHNOLOGY.
- Schlögl, Friedrich. "Chemical reaction models for non-equilibrium phase transitions." Zeitschrift für Physik 253.2 (1972): 147–161.
- Levenspiel, Octave. Chemical reaction engineering. Vol. 2. New York etc.: Wiley, 1972.