Hemangioma
| Hemangioma | |
|---|---|
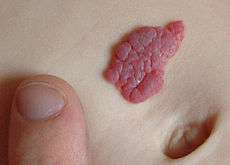 A small hemangioma of infancy | |
| Classification and external resources | |
| Specialty | Dermatology |
| ICD-10 | D18.0 (ILDS D18.010) |
| ICD-9-CM | 228.0 |
| ICD-O | M9120/0 |
| DiseasesDB | 30033 |
| MedlinePlus | 001459 |
| eMedicine | derm/201 |
| MeSH | D006391 |
A hemangioma is a benign and usually self-involuting tumor (swelling or growth) of the endothelial cells that line blood vessels that is characterised by increased number of normal or abnormal vessels filled with blood. It usually appears in the first weeks of life and grows most rapidly over the first six months. Usually, growth is complete and involution has commenced by twelve months. Half of all infantile hemangiomas have completed involution by age five, 70% by age seven, and most of the remainder by age twelve.[1] In more severe cases hemangiomas may leave residual tissue damage. In infancy, it is the most common tumor.[2] The word "hemangioma" comes from the Greek haema- (αίμα), "blood"; angeio (αγγείο), "vessel"; -oma (-ωμα), "tumor".
Terminology
The terminology used to define, describe and categorize vascular anomalies, abnormal lumps made up of blood vessels, has changed. The term hemangioma originally described any vascular tumor-like structure, whether it was present at or around birth or appeared later in life. Mulliken et al. categorized these conditions into two families: one of self-involuting tumors, growing lesions that eventually disappear, and another of malformations, enlarged or abnormal vessels present at birth and essentially permanent. The importance of this distinction is that it makes it possible for early-in-life differentiation between lesions that will resolve versus those that are permanent. Examples of permanent malformations include port-wine stains (capillary vascular malformation) and masses of abnormal swollen veins (venous malformations).[3] The Mulliken categorisation has received major confirmation following discovery of the Glut-1 marker.
Signs and symptoms
Hemangiomas are connected to the circulatory system. The appearance depends on location.
If they are on the surface of the skin, they are reminiscent of a ripe strawberry (hence, they are sometimes referred to as "strawberry hemangiomas"). If they are just under the skin they present as a bluish swelling. Sometimes they grow in internal organs such as the liver, larynx, or small and large intestines.[4] In most cases, hemangiomas will disappear over time. Some are formed during gestation and are called congenital hemangiomas; the most common (infantile hemangiomas) appear during the first few weeks of life. Infantile hemangioma is often initially misdiagnosed as a scratch or bruise; the correct diagnosis becomes obvious with further growth. Typically, at the earliest phase in a superficial lesion, one will see a bluish red area with obvious blood vessels and surrounding pallor. Sometimes they present as a flat red or pink area. Hemangiomas are the most common childhood tumor, occurring in approximately ten percent of Caucasians, and are less prevalent in other ethnicities. Females are three to five times as likely to have hemangiomas as males.
Hemangiomas are more common in twin pregnancies and low-birth-weight premature infants.[5] Approximately 80% are on the face and neck, or on the legs and arms; with the next most prevalent location being the liver. In infants, they may appear soon after birth and are different from other vascular abnormalities present. They grow to around 80% of their maximum size in the first 3 months and most reach maximum size at around 5 months.[6]
-
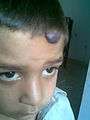
Hemangioma on forehead showing signs of early regression
-
Hemangioma on the scalp of a two-year-old child, in the "rest stage"
-
Hemangioma of the liver as seen on ultrasound
-
A liver hemangioma as seen on CT
Causes
The cause of hemangioma is currently unknown; however, several studies have suggested the importance of estrogen signaling in hemangioma proliferation. In 2007, a paper from the Stanford Children's Surgical Laboratory revealed that localized soft tissue hypoxia coupled with increased circulating estrogen after birth may be the stimulus.[7]
There is also a hypothesis presented by researchers at Harvard and the University of Arkansas that maternal placenta embolizes to the fetal dermis during gestation resulting in hemangiomagenesis.[8][9] However, researchers at Duke University conducted genetic analyses of single-nucleotide polymorphism in hemangioma tissue compared to the mother's DNA that contradicted this hypothesis.[10] More research is required to fully understand the explosive nature of hemangioma growth, which will hopefully yield targeted therapeutics to treat its most complicated presentations.
Diagnosis

Under the microscope, hemangiomas are unencapsulated aggregates of closely packed, thin-walled capillaries, usually with endothelial lining. Blood-filled vessels are separated by scant connective tissue. Their lumens may be thrombosed and organized. Hemosiderin pigment due to vessel rupture sometimes can be noticed.[11] Glut1 is a histochemical marker, highly specific for haemangioma and can be used to differentiate haemangioma from vascular malformations.[12]
Types
Hemangioma are of four types based on the type of vessel involved.
- Superficial
- Deep
- internal
- Mixed (containing both a superficial and a deep component)[13]
Complications
The vast majority of hemangiomas are not associated with complications. Hemangiomas may break down on the surface, called ulceration. If the ulceration is deep, significant bleeding may occur in rare occasions. Ulceration on the deeper area can be painful and problematic. If a hemangioma develops in the larynx, breathing can be compromised. A hemangioma can grow and block one of the eyes, causing an occlusion amblyopia. Very rarely, extremely large hemangiomas can cause high-output heart failure due to the amount of blood that must be pumped to excess blood vessels. Lesions adjacent to bone can also cause erosion of the bone.
The most frequent complaints about hemangiomas, however, stem from psychosocial complications: The condition can affect a person's appearance and can provoke attention and malicious reactions from others. Particular problems occur if the lip or nose is involved, as distortion can be difficult to treat surgically. The potential for psychological injury develops from school age onward. It is therefore important to consider treatment before school if adequate spontaneous improvement has not occurred.
Children with large segmental hemangiomas of the head and neck can be associated with a disorder called PHACES syndrome.[14][15]
Most hemangiomas disappear without treatment, leaving minimal or no visible marks. This may take many years. Large hemangiomas can leave visible skin changes secondary to severe stretching of the skin or damage to surface texture.
When hemangiomas interfere with vision, breathing, or threaten significant cosmetic injury (facial lesions and, in particular, nose and lips), they are usually treated. Treatment should be sought as soon as possible for a successful outcome. Between November 2009 and July 2013 the Children's Hospital of Minnesota treated over 180 children with a 98% success rate using the blood pressure medication propranolol. Effectiveness of propranolol is highest within the first 6 months of the onset of hemangioma. The treatment was first discovered in 2008 by a French group and has proven to show high success rates.[16]
Until recently, the mainstay of treatment was oral corticosteroid therapy, but there are now alternative treatments. A randomized trial showed that the beta-blocker propranolol reduced severe hemangiomas in infants.[17] The topically applied beta blocker solution/gel Timolol is being trialed for small facial hemangiomas that do not justify systemic treatment.[18] Other treatments include interferon[19] or vincristine. They may be considered if first-line therapy fails.
Surgical removal is sometimes indicated, particularly if there has been delay in commencing treatment and structural changes have become irreversible. Surgery may also be necessary to correct distortion of facial features, again in the case of inadequate or failed early medical intervention. Blockage of the airway will often require a tracheostomy: the insertion of an external airway through the front of the neck into the trachea below the level of the obstruction.
Smaller raised lesions are sometimes treated with injection of corticosteroid directly into the lesion. A pulsed dye laser can be useful for very early, flat, superficial lesions, if they appear in cosmetically significant areas or for those lesions that leave residual surface blood vessels in the case of incomplete resolution.[20] Sometimes a pulsed dye laser can be used to accelerate healing.
Raised lesions or lesions under the skin do not respond to laser treatment. Ulceration will usually heal with topical medication and special dressings under medical supervision. Applying pressure is not beneficial nor recommended.
Prognosis
Hemangiomas go through three stages of development and decay:
- In the proliferation stage, a hemangioma grows very quickly. This stage can last up to twelve months.
- In the rest stage, there is very little change in a hemangioma's appearance. This usually lasts until the infant is one to two years old.
- In the involution phase, a hemangioma begins to diminish in size. 50% of lesions will have disappeared by 5 years of age, and the vast majority will have gone by 10.
In some cases, the hemangioma doesn't diminish.
Notes
- ↑ Bowers RE, Graham EA, Tomlinson KM. "The natural history of the strawberry nevus." Archives of Dermatology. 1960;82:667; cited in Antaya RJ. "Infantile Hemangioma Clinical Presentation." Medscape. 2013
- ↑ Haggstrom AN, Drolet BA, Baselga E, et al. (September 2006). "Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment". Pediatrics 118 (3): 882–7. doi:10.1542/peds.2006-0413. PMID 16950977.
- ↑ Mulliken JB, Glowacki J (March 1982). "Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics". Plast. Reconstr. Surg. 69 (3): 412–22. doi:10.1097/00006534-198203000-00002. PMID 7063565.
- ↑ http://www.lacolon.com/patient-education/gi-associated-hemangiomas-and-vascular-malformations
- ↑ http://www.cincinnatichildrens.org/health/h/hemangioma/
- ↑ Oakley, Amanda. "Infantile haemangioma.". DermNet NZ. Retrieved February 11, 2013. External link in
|publisher=(help) - ↑ Kleinman ME, Greives MR, Churgin SS, et al. (December 2007). "Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma". Arterioscler. Thromb. Vasc. Biol. 27 (12): 2664–70. doi:10.1161/ATVBAHA.107.150284. PMID 17872454.
- ↑ Barnés CM, Huang S, Kaipainen A, et al. (December 2005). "Evidence by molecular profiling for a placental origin of infantile hemangioma". Proc. Natl. Acad. Sci. U.S.A. 102 (52): 19097–102. doi:10.1073/pnas.0509579102. PMC 1323205. PMID 16365311.
- ↑ North PE, Waner M, Brodsky MC (April 2002). "Are infantile hemangiomas of placental origin?". Ophthalmology 109 (4): 633–4. doi:10.1016/S0161-6420(02)01071-0. PMID 11949625.
- ↑ Pittman KM, Losken HW, Kleinman ME, et al. (November 2006). "No evidence for maternal-fetal microchimerism in infantile hemangioma: a molecular genetic investigation". J. Invest. Dermatol. 126 (11): 2533–8. doi:10.1038/sj.jid.5700516. PMID 16902414.
- ↑ Kumar Vinay, Robbins and Coltran pathologic basis of disease 8ed. pp. 520-521 Philadelphia: Saunders Elsevier, 2010. ISBN 978-0-8089-2402-9
- ↑ North PE, Waner M, Mizeracki A, Mihm MC Jr. (Jan 2000). "GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas.". Hum Pathology 31 (1): 11–22. doi:10.1016/S0046-8177(00)80192-6. PMID 10665907.
- ↑ dermnetnz.org/vascular/haemangioma.html
- ↑ Oza VS, Wang E, Berenstein A, et al. (April 2008). "PHACES association: a neuroradiologic review of 17 patients". AJNR Am J Neuroradiol 29 (4): 807–13. doi:10.3174/ajnr.A0937. PMID 18223093.
- ↑ Heyer GL, Dowling MM, Licht DJ, et al. (February 2008). "The cerebral vasculopathy of PHACES syndrome". Stroke 39 (2): 308–16. doi:10.1161/STROKEAHA.107.485185. PMID 18174492.
- ↑ Hunzeker C, Geronemus R (2010). ") "Treatment of Superficial Infantile Hemangiomas of the Eyelid Using the 595-nm Pulsed Dye Laser" (PDF). Dermatol. Surg. 36 (5): 590–597. doi:10.1111/j.1524-4725.2010.01511.x. PMID 20384759.
- ↑ Hogeling M; et al. (August 2011). "A randomized controlled trial of propranolol for infantile hemangiomas". Pediatrics 128 (2): e259–e266. doi:10.1542/peds.2010-0029. PMID 21788220.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help) - ↑ Guo S, Ni N (February 2010). "Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution". Arch. Ophthalmol. 128 (2): 255–6. doi:10.1001/archophthalmol.2009.370. PMID 20142555.; Pope E, Chakkittakandiyil A (May 2010). "Topical timolol gel for infantile hemangiomas: a pilot study". Arch Dermatol 146 (5): 564–5. doi:10.1001/archdermatol.2010.67. PMID 20479314.
- ↑ Wilson MW, Hoehn ME, Haik BG, Rieman M, Reiss U (May 2007). "Low-dose cyclophosphamide and interferon alfa 2a for the treatment of capillary hemangioma of the orbit". Ophthalmology 114 (5): 1007–11. doi:10.1016/j.ophtha.2006.11.031. PMID 17337066.
- ↑ Rizzo C, Brightman L, Chapas A, Hale E, Cantatore-Francis J, Bernstein L, Geronemus R (2009). "Outcomes of Childhood Hemangiomas Treated with the Pulsed Dye Laser with Dynamic Cooling: A Retrospective Chart Analysis" (PDF). Dermatol. Surg. 35 (12): 1947–1954. doi:10.1111/j.1524-4725.2009.01356.x. PMID 19889007.
External links
- Hemangioma Investigator Group
- Hemangioma - Children's Hospital Los Angeles
- Hemangioma - at Web MD
- Hemangioma - Children's Hospital Boston
- Humpath #1990 (Pathology images) at humpath.com
- MedlinePlus Encyclopedia Hemangioma
- Hepatic Hemangioma at medicinenet.com
- Haemangiomas of infancy at rch.org.au
- Liver Hemangioma at USUHS - MedPix
- Infantile Hemangiomas - Cincinnati Children's Hospital
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||