Cefprozil
 | |
| Systematic (IUPAC) name | |
|---|---|
|
7-[2-amino-2-(4-hydroxyphenyl)-acetyl]amino-8-oxo-3-prop-1-enyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Clinical data | |
| Trade names | Cefzil |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a698022 |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Protein binding | 36% |
| Biological half-life | 1.3 hours |
| Identifiers | |
| CAS Number |
92665-29-7 |
| ATC code | J01DC10 |
| PubChem | CID 9887643 |
| DrugBank |
DB01150 |
| ChemSpider |
8063315 |
| UNII |
1M698F4H4E |
| KEGG |
D07651 |
| ChEBI |
CHEBI:3506 |
| ChEMBL |
CHEMBL1742 |
| Chemical data | |
| Formula | C18H19N3O5S |
| Molar mass | 389.427 g/mol |
| |
| |
| | |
Cefprozil, sometimes spelled cefproxil and marketed under the trade name Cefzil, is a second-generation cephalosporin type antibiotic. In Europe it is marketed using the trade names Procef and Cronocef. It can be used to treat bronchitis, ear infections, skin infections, and other bacterial infections. It comes as a tablet and as a liquid suspension.
Although there is a widely quoted cross-allergy risk of 10% between cephalosporins and penicillin, an article in the Journal of Family Practice[1] has shown no increased risk for cross-allergy for cefprozil and several other second-generation or later cephalosporins.
Spectrum of bacterial susceptibility and resistance
Currently bacteria like Enterobacter aerogenes, Morganella morganii and Pseudomonas aeruginosa are resistant to Cefprozil, while Salmonella Agona and Streptococci are susceptible to Cefprozil. Some of the bacteria like Brucella abortus, Moraxella catarrhalis and Streptococcus pneumonia have developed resistance towards Cefprozil in varying degrees. For detailed minimum inhibition concentration information, please refer to Cefprozil Susceptibility and Resistance Data sheet.[2]
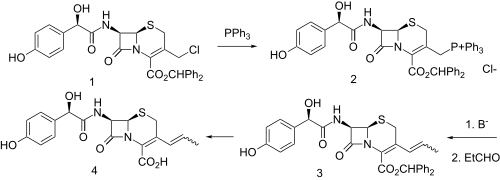
Synthesis
Displacement of the allylic chloride in intermediate (1) with triphenylphosphine gives the phosphonium salt (2). This functionality is then converted to its ylide; condensation with acetaldehyde then leads to the vinyl derivative (3); deprotection then gives cefprozil. Semisynthetic oral cephalosporin consisting of ~90:10 Z/E isomeric mixture.
References
- ↑ Pichichero, ME (February 2006). "Cephalosporins can be prescribed safely for penicillin-allergic patients". J Fam Pract 55 (2): 106–12. PMID 16451776.
- ↑ "Cefprozil Susceptibility and Resistance Data" (PDF). Retrieved 23 July 2013.
- ↑ H. Hoshi et al., DE 3402642; eidem, U.S. Patent 4,520,022 (1984, 1985 both to Bristol-Myers).
- ↑ Naito, T; Hoshi, H; Aburaki, S; Abe, Y; Okumura, J; Tomatsu, K; Kawaguchi, H (1987). "Synthesis and structure-activity relationships of a new oral cephalosporin, BMY-28100 and related compounds". The Journal of antibiotics 40 (7): 991–1005. PMID 3624077.
- ↑ M. A. Kaplan et al., U.S. Patent 4,727,070 (1988 to Bristol-Myers).
External links
- Cefprozil MedlinePlus Drug Information
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||