Complex regional pain syndrome
| Complex regional pain syndrome | |
|---|---|
 Reflex sympathetic dystrophy | |
| Classification and external resources | |
| Specialty | Neurology |
| ICD-10 | M89.0, G56.4 |
| ICD-9-CM | 337.21, 337.22, 354.4, 355.71 |
| DiseasesDB | 12635 16345 |
| MedlinePlus | 007184 |
| eMedicine | pmr/123 |
| Patient UK | Complex regional pain syndrome |
| MeSH | D020918 |
Complex regional pain syndrome (CRPS) formerly reflex sympathetic dystrophy (RSD), "causalgia", or reflex neurovascular dystrophy (RND) is an amplified musculoskeletal pain syndrome (AMPS). It is a chronic systemic disease characterized by severe pain, swelling, and changes in the skin. CRPS often worsens over time. It may initially affect an arm or leg and spread throughout the body; 35% of people report symptoms throughout their whole body.[1] Other potential effects include: systemic autonomic dysregulation; neurogenic edema; musculoskeletal, endocrine, or dermatological manifestations; and changes in urological or gastrointestinal function.[2]
The cause of CRPS is unknown though CRPS is associated with dysregulation of the central nervous system[3] and autonomic nervous system resulting in multiple functional loss, impairment, and disability. Precipitating factors include injury and surgery, although there are documented cases where no injury had occurred at the original site. CRPS is not caused by psychological factors, yet the constant pain and reduced quality of life has been known to cause psychological problems (such as increased depression and anxiety). Although "research does not reveal support for specific personality or psychopathology predictors of the condition," CRPS is associated with psychosocial effects, including impaired social and occupational function.[4] Treatment is complicated, involving medications, physical therapy, psychological treatments, and neuromodulation and is often unsatisfactory, especially if delayed.[5]
Classification
- Type I, formerly known as reflex sympathetic dystrophy (RSD), Sudeck's atrophy, or algoneurodystrophy, does not exhibit demonstrable nerve lesions. As the vast majority of patients diagnosed with CRPS have this type, it is most commonly referred to in medical literature as Type I.
- Type II, formerly known as causalgia, has evidence of obvious nerve damage. Type II CRPS tends to feature the more painful and difficult-to-control symptomes of CRPS; The Type II disease scores 47 out of 50 on the McGill pain scale[6] (however there are seemingly little or no data pertaining to Type I specifically here). In Type II the "cause" of the syndrome is a known or obvious nerve injury, although the cause of the mechanisms of CRPS Type II are as unknown as the mechanisms of Type I.
Signs and symptoms

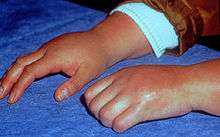
Clinical features of CRPS have been found to be neurogenic inflammation, nociceptive sensitisation, vasomotor dysfunction, and maladaptive neuroplasticity.[7] The symptoms of CRPS usually initially manifest near the site of a, typically minor, injury. The most common symptoms are pain sensations, including burning, stabbing, grinding, and throbbing. Moving or touching the limb is often intolerable. The patient may also experience muscle spasms, local swelling, sensitivity to things such as water, touch, and vibrations, abnormally increased sweating, changes in skin temperature (usually hot but sometimes cold) and color (bright red or a reddish violet), softening and thinning of bones, joint tenderness or stiffness, and/or restricted or painful movement. Falls, pre-syncope, and syncope (fainting) are infrequently reported, as are visual problems. Regional Osteoporosis is possible. The symptoms of CRPS vary in severity and duration. Since CRPS is a systemic problem, potentially any organ can be affected.
The pain of CRPS is continuous, and it is widely recognised that it can be heightened by emotional or physical stress.[8]
Complex regional pain syndrome's cause is unknown. Nerve damage is considered to be the cause in many cases. Evidence of nerve damage as the trigger for CRPS is supported by observations that the disruption of certain nerve relationships to pain signaling result in improvements for patients.
Patients are frequently classified into two groups based upon temperature: "warm" or "hot" CRPS in one group and "cold" CRPS in the other group. The majority of patients (approximately 70%) have the "hot" type, which is said to be an acute form of CRPS.[9] Cold CRPS is said to be indicative of a more chronic CRPS and is associated with poorer McGill Pain Questionnaire (MPQ) scores, increased central nervous system involvement, and a higher prevalence of dystonia.[9] Prognosis is not favourable for cold CRPS patients; longitudinal studies suggest these patients have "poorer clinical pain outcomes and show persistent signs of central sensitisation correlating with disease progression".[10]
Previously it was considered that CRPS had three stages; it is now believed that patients with CRPS do not progress through these stages sequentially. These stages may not be time-constrained and could possibly be event-related, such as ground-level falls or re-injuries of previously damaged areas. thus, rather than a progression of CRPS from bad to worse, it is now thought, instead, that patients are likely to have one of the three following types of disease progression:
- "Stage" one is characterized by severe, burning pain at the site of the injury, muscle spasms, joint stiffness, restricted mobility, rapid hair and nail growth, and vasospasm. The vasospasm is that which causes the changes in the color and temperature of the skin. Some may experience hyperhydrosis (increased sweating). In mild cases this stage lasts a few weeks, in which it can subside spontaneously or respond rapidly to treatment (physical therapy, pain specialist).
- "Stage" two is characterized by more intense pain. Swelling spreads, hair growth diminishes, nails become cracked, brittle, grooved, and spotty, osteoporosis becomes severe and diffuse, joints thicken, and muscles atrophy.
- "Stage" three is characterized by irreversible changes in the skin and bones, while the pain becomes unyielding and may involve the entire limb. There is marked muscle atrophy, severely limited mobility of the affected area, and flexor tendon contractions (contractions of the muscles and tendons that flex the joints). Occasionally the limb is displaced from its normal position, and marked bone softening and thinning is more dispersed.
Cause
Investigators estimate that 2-5% of those with peripheral nerve injury,[11] and 13-70 percent of those with hemiplegia (paralysis of one side of the body),[12] will develop CRPS. In addition, some studies have indicated that cigarette smoking was strikingly present in patients and is statistically linked to RSD. In one study, 68% of patients versus 37% of hospitalized controls were found. This may be involved in its pathology by enhancing sympathetic activity, vasoconstriction, or by some other unknown neurotransmitter-related mechanism.[13] 7% of people who have CRPS in one limb later develop it in another limb.
Pathophysiology
Complex regional pain syndrome is a multifactorial disorder with clinical features of neurogenic inflammation (swelling in the central nervous system), nociceptive sensitisation (which causes extreme sensitivity or allodynia), vasomotor dysfunction (blood flow problems which cause swelling and discolouration) and maladaptive neuroplasticity (where the brain changes and adapts with constant pain signals); CRPS is the result of an "aberrant [inappropriate] response to tissue injury".[7] The "underlying neuronal matrix" of CRPS is seen to involve cognitive and motor as well as nociceptive processing; pinprick stimulation of a CRPS affected limb was painful (mechanical hyperalgesia) and showed a "significantly increased activation" of not just the S1 cortex (contralateral), S2 (bilateral) areas, and insula (bilateral) but also the associative-somatosensory cortices (contralateral), frontal cortices, and parts of the anterior cingulate cortex.[14] In contrast to previous thoughts reflected in the name RSD, it appears that there is reduced Sympathetic Nervous System outflow, at least in the affected region (although there may be sympatho-afferent coupling).[15] Wind-up (the increased sensation of pain with time)[16] and central nervous system (CNS) sensitization are key neurologic processes that appear to be involved in the induction and maintenance of CRPS.[17]
There is compelling evidence that the N-methyl-D-aspartate (NMDA) receptor has significant involvement in the CNS sensitization process.[18] It is also hypothesized that elevated CNS glutamate levels promote wind-up and CNS sensitization.[17] In addition, there is experimental evidence that demonstrates NMDA receptors in peripheral nerves.[19] Because immunological functions can modulate CNS physiology, it has also been hypothesized that a variety of immune processes may contribute to the initial development and maintenance of peripheral and central sensitization.[20][21] Furthermore, trauma related cytokine release, exaggerated neurogenic inflammation, sympathetic afferent coupling, adrenoreceptor pathology, glial cell activation, cortical reorganisation,[22] and oxidative damage (e.g., by free radicals) are all concepts that have been implicated in the pathophysiology of CRPS.[23]
The pathophysiology of complex regional pain syndrome has not yet been defined; there is conjecture that CRPS, with its variable manifestations, could be the result of multiple pathophysiologies.[15]
Diagnosis
CRPS types I and II share the common diagnostic criteria shown below. Spontaneous pain or allodynia (pain resulting from a stimulus which would not normally provoke pain, such as a light touch of the skin) is not limited to the territory of a single peripheral nerve and is disproportionate to the inciting event.
- There is a history of edema, skin blood flow abnormality, or abnormal sweating in the region of the pain since the inciting event.
- No other conditions can account for the degree of pain and dysfunction.
The two types differ only in the nature of the inciting event. Type I CRPS develops following an initiating noxious event that may or may not have been traumatic, while type II CRPS develops after a nerve injury.
No specific test is available for CRPS, which is diagnosed primarily through observation of the symptoms. However, thermography, sweat testing, x-rays, electrodiagnostics, and sympathetic blocks can be used to build up a picture of the disorder. Diagnosis is complicated by the fact that some patients improve without treatment. A delay in diagnosis and/or treatment for this syndrome can result in severe physical and psychological problems. Early recognition and prompt treatment provide the greatest opportunity for recovery.
The International Association for the Study of Pain (IASP) lists the diagnostic criteria for complex regional pain syndrome I (CRPS I) (RSDS) as follows:
- The presence of an initiating noxious event or a cause of immobilization
- Continuing pain, allodynia (perception of pain from a nonpainful stimulus), or hyperalgesia (an exaggerated sense of pain) disproportionate to the inciting event
- Evidence at some time of edema, changes in skin blood flow, or abnormal sudomotor activity in the area of pain
- The diagnosis is excluded by the existence of any condition that would otherwise account for the degree of pain and dysfunction.
According to the IASP, CRPS II (causalgia) is diagnosed as follows:
- The presence of continuing pain, allodynia, or hyperalgesia after a nerve injury, not necessarily limited to the distribution of the injured nerve
- Evidence at some time of edema, changes in skin blood flow, or abnormal sudomotor activity in the region of pain
- The diagnosis is excluded by the existence of any condition that would otherwise account for the degree of pain and dysfunction.
The IASP criteria for CRPS I diagnosis has shown a sensitivity ranging from 98–100% and a specificity ranging from 36–55%. Per the IASP guidelines, interobserver reliability for CRPS I diagnosis is poor. Two other criteria used for CRPS I diagnosis are Bruehl's criteria and Veldman's criteria, which have moderate to good interobserver reliability. In the absence of clear evidence supporting one set of criteria over the other, clinicians may use IASP, Bruehl’s, or Veldman’s clinical criteria for diagnosis. While the IASP criteria are nonspecific and possibly not as reproducible as Bruehl’s or Veldman’s criteria, they are cited more widely in literature, including treatment trials.[24]
Thermography
Presently, established empirical evidence suggests against thermography's efficacy as a reliable tool for diagnosing CRPS. Although CRPS may, in some cases, lead to measurably altered blood flow throughout an affected region, many other factors can also contribute to an altered thermographic reading, including the patient's smoking habits, use of certain skin lotions, recent physical activity, and prior history of trauma to the region. Also, not all patients diagnosed with CRPS demonstrate such "vasomotor instability" — less often, still, those in the later stages of the disease.[25] Thus, thermography alone cannot be used as conclusive evidence for - or against - a diagnosis of CRPS and must be interpreted in light of the patient's larger medical history and prior diagnostic studies.[26]
In order to minimise the confounding influence of external factors, patients undergoing infrared thermographic testing must conform to special restrictions regarding the use of certain vasoconstrictors (namely, nicotine and caffeine), skin lotions, physical therapy, and other diagnostic procedures in the days prior to testing. Patients may also be required to discontinue certain pain medications and sympathetic blockers. After a patient arrives at a thermographic laboratory, he or she is allowed to reach thermal equilibrium in a 16–20 °C, draft-free, steady-state room wearing a loose fitting cotton hospital gown for approximately twenty minutes. A technician then takes infrared images of both the patient's affected and unaffected limbs, as well as reference images of other parts of the patient's body, including his or her face, upper back, and lower back. After capturing a set of baseline images, some labs further require the patient to undergo cold-water autonomic-functional-stress-testing to evaluate the function of his or her autonomic nervous system's peripheral vasoconstrictor reflex. This is performed by placing a patient's unaffected limb in a cold water bath (approximately 20 °C) for five minutes while collecting images. In a normal, intact, functioning autonomic nervous system, a patient's affected extremity will become colder. Conversely, warming of an affected extremity may indicate a disruption of the body's normal thermoregulatory vasoconstrictor function, which may sometimes indicate underlying CRPS.[27]
Radiography
Scintigraphy, plain radiographs, and magnetic resonance imaging (MRI) may all be useful diagnostically. Patchy osteoporosis (post-traumatic osteoporosis), which may be due to disuse of the affected extremity, can be detected through X-ray imagery as early as two weeks after the onset of CRPS. A bone scan of the affected limb may detect these changes even sooner. Bone densitometry can also be used to detect changes in bone mineral density. It can also be used to monitor the results of treatment since bone densitometry parameters improve with treatment.
Electrodiagnostic testing
Electromyography (EMG) and Nerve Conduction Studies (NCS) are important ancillary tests in CRPS because they are among the most reliable methods of detecting nerve injury. They can be used as one of the primary methods to distinguish between CRPS I & II, which differ based on whether there is evidence of actual nerve damage. EMG & NCS are also among the best tests for ruling in or out alternative diagnoses. CRPS is a "diagnosis of exclusion", which requires that there be no other diagnosis that can explain the patient's symptoms. This is very important to emphasise because otherwise patients can be given a wrong diagnosis of CRPS when they actually have a treatable condition that better accounts for their symptoms. An example is severe Carpal Tunnel Syndrome, which can often present in a very similar way to CRPS. Unlike CRPS, Carpal Tunnel Syndrome can often be corrected with surgery in order to alleviate the pain and avoid permanent nerve damage and malformation.[28]
Both EMG and NCS involve some measure of discomfort. EMG involves the use of a tiny needle that is inserted into specific muscles to test the associated muscle and nerve function. Both EMG & NCS involve very mild shocks that in normal patients are comparable to a rubber band snapping on the skin. Although these tests can be very useful in CRPS, thorough informed consent needs to be obtained prior to the procedure, particularly in patients experiencing severe allodynia. In spite of the utility of the test, these patients may wish to decline the procedure in order to avoid discomfort.
Prevention
Vitamin C may be useful in prevention of the syndrome following fracture of the forearm.[29]
Treatment
The general strategy in CRPS treatment is often multi-disciplinary, with the use of different types of medications combined with distinct physical therapies.
Physical therapy
Physical and occupational therapy are important components of the management of CRPS primarily by desensitising the affected body part, restoring motion, and improving function. Physical therapy interventions for CRPS can include specific modalities such as transcutaneous electrical nerve stimulation, progressive weight bearing, tactile desensitization, massage, and contrast bath therapy. These interventions tailored specifically to each individual person can be used to improve pain and function to help people return to normal activities of daily living.[30] Some people at certain stages of the disease are incapable of participating in physical therapy due to touch intolerance. This may be where Graded Motor Imagery and Mirror Therapy (see below) are particularly helpful. People with CRPS often develop guarding behaviors where they avoid using or touching the affected limb. This inactivity exacerbates the disease and perpetuates the pain cycle. Therefore, optimizing the multimodal treatment is paramount to allow for use of the involved body part. Physical therapy works best for most patients, especially goal-directed therapy, where the patient begins from an initial point, regardless of how minimal, and then endeavors to increase activity each week. Therapy is directed at facilitating the patient to engage in physical therapy, movement, and stimulation of the affected areas. One difficulty with the idea of physical therapy, however, is that it means different things to different people. There is one systematic review of the use of physical and occupational therapy for the treatment of CRPS.[31] That review concluded, "Narrative synthesis of the results, based on effect size, found there was good to very good quality level II evidence that graded motor imagery is effective in reducing pain in adults with CRPS-1, irrespective of the outcome measure used. No evidence was found to support treatments frequently recommended in clinical guidelines, such as stress loading. CONCLUSIONS: Graded motor imagery should be used to reduce pain in adult CRPS-1 patients."[31]
Physical therapy has been used under light general anesthesia in an attempt to remobilize the extremity. Such remobilization is used cautiously to avoid damage to atrophied tissue and bones that have become osteodystrophic.
Although there is no denying the importance of a multidisciplinary approach in the management of CRPS,[32] recent research suggests that physical therapy intervention may be successful in decreasing symptoms of CRPS without the use of medications. “Pain exposure” physical therapy (PEPT) is based on the premise that pain may be exacerbated and maintained by psychosocial and behavioural factors, and therefore, these factors must be addressed as a component of CRPS management. PEPT combines a progressive loading exercise program with pain-avoidance behaviour management. Progressive loading (i.e., loading extremities beyond limit of pain) includes passive and active exercises to mobilize joints and muscle stretching and is believed to reduce sensitization (both central and peripheral) and may also restore autonomic deregulation and cortical representation in CRPS. As the name suggests, pain avoidance behaviour management attempts to reduce behaviours that maintain disuse and pain avoidance (e.g., kinesiophobia, pain avoidance and learned non-use, and pain catastrophizing), with the goal of increasing self-confidence in the individual’s physical capabilities.[33]
A recent multiple single-case design study by Van de Meent et al. (2011)[33] found PEPT to be a safe and effective method of treatment for individuals with CRPS. Results showed improvements on a variety of outcomes measures, including pain intensity, kinesiophobia, muscle strength, arm/shoulder/hand disability, walking speed, and perceived health. However, although these results are promising, this is a relatively new topic of study and more research needs to be done in the area.
In children, CRPS is often treated with an intense physical and occupational therapy program for amplified musculoskeletal pain syndromes. These programs also employ counseling, art therapy, and music therapy. These evidence-based programs report long-term pain resolution success rates as high as 88%.[34] In one study of 103 children with CRPS, 92% finished such a program pain-free and 88% were pain-free five years later at the time of follow-up.[35] The efficacy of such a program has not been studied in adults.
Mirror box therapy
Mirror box therapy uses a mirror box, or a stand-alone mirror, to create a reflection of the normal limb such that the patient thinks they are looking at the affected limb. Movement of this reflected normal limb is then performed so that it looks to the patient as though they are performing movement with the affected limb (although it will be pain free due to the fact it is a normal limb being reflected).
Mirror box therapy appears to be beneficial in early CRPS (McCabe et al., 2003b);.[36] However, Lorimer Moseley (University of South Australia) has cautioned that the beneficial effects of mirror therapy for CRPS are still unproven.[37] Importantly, the precise neural mechanisms of action are unknown and need to be studied using a combination of behavioural and neuroimaging approaches.[38]
Graded motor imagery
Because studies have shown that problems in the primary motor cortex are found in patients who suffer from CRPS, treatments have been developed that focus on normalizing motor representations in that part of the brain. Graded motor imagery[39][40] has now been tested in three[41][42][43] randomised controlled trials and has shown to be effective at reducing pain and disability in people with chronic CRPS or phantom limb pain after amputation or avulsion injury of the brachial plexus.
Graded motor imagery is a sequential process that consists of (a) laterality reconstruction, (b) motor imagery, and (c) mirror therapy.[32][44]
Tactile discrimination training
Another approach to CRPS is based on a treatment called sensory discrimination training, which was used for phantom limb pain. A randomised controlled trial [45] demonstrated a significant drop in pain after ten days training. For CRPS, a replicated case series [37] and a randomised repeated measures experiment [46] both demonstrated an effect of tactile discrimination training on pain, disability, and sensory function in people with CRPS of various durations. This treatment has not been tested in a randomised controlled trial.
Medications
Physicians use a variety of drugs to treat CRPS, including antidepressants, anti-inflammatories such as corticosteroids, COX-inhibitors such as piroxicam, bisphosphonates, vasodilators, GABA analogs such as gabapentin and pregabalin, alpha- or beta-adrenergic-blocking compounds, and the entire pharmacy of opioids. Occasional uses of Butorphanol also can be helpful during moments of heightened pain. In a limited study, Low-dose naltrexone has been found to be effective in the remittance of many prominent symptoms, including dystonic spasms and fixed dystonia (respectively).[47]
Ketamine
Ketamine, a dissociative anesthetic, appears promising as a treatment for complex regional pain syndrome.[48] It may be used in low doses if other treatments have not worked.[49][50] No benefit on either function or depression; however, has been seen.[50]
Bisphosphonate treatment
Bisphosphonates were described in 2009 as "among the only class of medications that has survived placebo-controlled studies showing statistically significant improvement (in CRPS) with therapy."[51] Researchers in the 2000s used steadily increased dosages and experimented with other bisphosphonates to improve results, increase response, and put higher percentages of patients treated into long-term remission.[52][53]
In Varenna et al.'s 2000 study, patients who had CRPS for up to a year were dosed with 300 mg infusions of clodronate on ten consecutive days (3000 mg total). The group receiving clodronate reported pain falling significantly in the first 40 days and average pain in the group was reported as being close to zero 180 days following treatment. For Varenna et al.'s 2013 study,[54] patients were given four infusions of 100 mg of intravenous neridronate within 4 to 10 days (400 mg total). The group receiving the neridronate infusions reported rapid reduction in pain levels.
In their 2004 paper "Efficacy of Pamidronate in Complex Regional Pain Syndrome Type I", Robinson et al. report that with single 60 mg infusions, "several patients with established disease of several years duration responded to pamidronate, perhaps reflecting the heterogeneity of this condition".[55]
The 2009 European Journal of Pain article "Bisphosphonates for the therapy of complex regional pain syndrome I - Systematic Review" by Florian Brunner et al. concluded that the "very limited data reviewed showed that bisphosphonates have the potential to reduce pain associated with bone loss in patients with CRPS I, however, at present there is not sufficient evidence to recommend their use in practice".[56] Varenna et al. report in their 2013 double blind placebo-controlled study of 80 patients that all 78 patients who participated in a lengthy follow-up were dramatically improved and continued to improve. The authors conclude that "These results provide conclusive evidence that the use of bisphosphonates, at appropriate doses, is the treatment of choice for CRPS-I".[54]
At the University of Verona, which participated in the 2013 Varenna et al. study, 7500 I.U. of Vitamin D per week are often given after treatment to help the bones heal. Varenna et al. states that the results are "conjectural, mainly because the exact pathophysiology of the disease is still unknown."[54]
Topical treatment
CRPS can also be treated with DMSO 50% cream.[57] A novel approach to treat CRPS is with the multimodal stepped care approach.[58] Step by step a topical analgesic will be tried to examine its effectiveness in reducing pain. When a topical analgesic has some pain reducing effects, though not completely, another topical analgesic from a different class can be added to enhance the pain reducing effects.[58] Usually one to four topical agents can be used simultaneously to get an optimal pain reducing effect. Combination therapy between ketamine cream and the anti-inflammatory palmitoylethanolamide seems worth mentioning.[59]
Local anaesthetic injections
Injection of a local anesthetic, such as lidocaine, is often the first step in treatment. Injections are repeated as needed. The results of local anesthetic injections are short lasting, and the procedure is risky. However, early intervention with non-invasive management may be preferred to repeated nerve blockade. The use of topical lidocaine patches has not been shown to be of use in the treatment of CRPS-1 and 2.
Botox injections
Intramuscular botulinum injections have been shown recently to benefit patients with CRPS symptoms localized to one extremity.[60] These injections may reduce the muscle spasm associated with CRPS and likely also decrease the inflammation associated with CRPS. The main advantages of this treatment are that it is relatively cheap, safe, and easy to administer. The major disadvantage is that it may need to be repeated after a few months.
Surgery
Spinal cord stimulators
Neurostimulation (spinal cord stimulator) may also be surgically implanted to reduce the pain by directly stimulating the spinal cord. These devices are surgically placed by trained physicians. An electrode is placed in the epidural space in the region of the spinal cord associated with the body part to be treated. Once placed, programming by a knowledgeable clinician will personalize the device to each patient's pain complaints for the optimal outcome. High frequencies are normally utilized for CRPS patients. A systematic review concluded that spinal cord stimulation appears to be an effective therapy in the management of patients with CRPS type I (Level A evidence) and type II (Level D evidence).[61] Moreover, there is evidence to demonstrate that SCS is a cost-effective treatment for CRPS type I.
A randomised controlled trial performed by Kemler et al. (2000) on spinal cord stimulation (SCS) in patients with refractory RSD demonstrated that the group receiving SCS + physical therapy (n=36) had a mean reduction of 2.4 cm (using Visual analogue scale) in the intensity of pain at six months compared to a mean increase of 0.2 cm in the group assigned to receive physical therapy alone (n=18). The intensity of pain was found to be statistically significantly different between the two groups (P < 0.001). In addition, a greater proportion of patients in the SCS + physical therapy reported a 6 (“much improved” outcome) based on a global perceived effect scale compared to physical therapy alone (39% vs. 6%, P = 0.01). However, the study did not find clinically significant improvement in functional status.[62]
Sympathectomy
Surgical, chemical, or radiofrequency sympathectomy — interruption of the affected portion of the sympathetic nervous system — can be used as a last resort in patients with impending tissue loss, edema, recurrent infection, or ischemic necrosis.[63] However, there is little evidence that these permanent interventions alter the pain symptoms of the affected patients, and in addition to the normal risks of surgery, such as bleeding and infection, sympathectomy has several specific risks, such as adverse changes in how nerves function.
Amputation
There is no randomized study in medical literature that has studied the response with amputation of patients who have failed the above-mentioned therapies and who continue to be miserable. Nonetheless, there are reports that on average cite about half of the patients will have resolution of their pain, while half will develop phantom limb pain and/or pain at the amputation site. It is likely that as in any other chronic pain syndrome, the brain becomes chronically stimulated with pain, and late amputation may not work as well as it might be expected. In a survey of fifteen patients with CRPS Type 1, eleven responded that their life was better after amputation.[64] Since this is the ultimate treatment of a painful extremity, it should be left as a last resort.
Adjunctive treatment
EEG Biofeedback,[65] various forms of psychotherapy,[66] relaxation techniques, and hypnosis [67] are adjunctive treatments that assist coping.
Prognosis
Good progress can be made in treating CRPS if treatment is begun early, ideally within three months of the first symptoms. If treatment is delayed, however, the disorder can quickly spread to the entire limb, and changes in bone, nerve, and muscle may become irreversible. The prognosis is not always good. Johns Hopkins Hospital reports that 77% of sufferers have spreads from the original site or flares in other parts of the body. The limb, or limbs, can experience muscle atrophy, loss of use, and functionally useless parameters that require amputation. RSD/CRPS will not "burn itself out", but if treated early, it is likely to go into remission. Once you are diagnosed with Complex Regional Pain Syndrome the likelihood of it resurfacing after going into remission is significant. It is important that you take precautions and seek immediate treatment upon any injury. Notify the treating physicians of your prior history of Complex Regional Pain Syndrome.
Epidemiology
CRPS can occur at any age with the average age at diagnosis being 42.[11] It affects both men and women; however, CRPS is three times more frequent in females than males.[11] It affects adults and children. The number of reported CRPS cases among adolescents and young adults is increasing.[68]
History
The condition currently known as CRPS was originally described during the American Civil War by Silas Weir Mitchell, who is sometimes also credited with inventing the name "causalgia."[69] However, this term was actually coined by Mitchell's friend Robley Dunglison from the Greek words for heat and for pain.[70] Contrary to what is commonly accepted, it emerges that these causalgias were certainly major by the importance of the vasomotor and sudomotor symptoms but stemmed from minor neurological lesions. Mitchell even thought that the CRPS etiology came from the cohabitation of the altered and unaltered cutaneous fibres on the same nerve distribution territory.[71] In the 1940s, the term reflex sympathetic dystrophy came into use to describe this condition, based on the theory that sympathetic hyperactivity was involved in the pathophysiology.[72] In 1959, Noordenbos observed in caulsalgia patients that "the damage of the nerve is always partial."[73] Misuse of the terms, as well as doubts about the underlying pathophysiology, led to calls for better nomenclature. In 1993, a special consensus workshop held in Orlando, Florida, provided the umbrella term "complex regional pain syndrome", with causalgia and RSD as subtypes.[74]
Society and culture
- Paula Abdul, Singer, Actor, TV Personality.[75]
- Jill Kinmont Boothe, US ski slalom champion.[76]
- Gemma Collis, British paralympic fencer.[77]
- Shin Dong-wook, South Korean actor and model.[78]
- Howard Hughes, American business tycoon, aviator, inventor, filmmaker and philanthropist.[79]
- Barby Ingle, Author, radio and television personality.[80]
- Rachel Morris, British paralympic cyclist.[81]
- Cynthia Toussaint, Author and media personality.[82]
- Danielle Brown, British paralympic archer.[83]
Research
The National Institute of Neurological Disorders and Stroke (NINDS), a part of the National Institutes of Health (NIH), supports and conducts research on the brain and central nervous system, including research relevant to RSDS, through grants to major medical institutions across the country. NINDS-supported scientists are working to develop effective treatments for neurological conditions and ultimately, to find ways of preventing them. Investigators are studying new approaches to treat CRPS and intervene more aggressively after traumatic injury to lower the patient's chances of developing the disorder. In addition, NINDS-supported scientists are studying how signals of the sympathetic nervous system cause pain in CRPS patients. Using a technique called microneurography, these investigators are able to record and measure neural activity in single nerve fibers of affected patients. By testing various hypotheses, these researchers hope to discover the unique mechanism that causes the spontaneous pain of CRPS, and that discovery may lead to new ways of blocking pain. Other studies to overcome chronic pain syndromes are discussed in the pamphlet "Chronic Pain: Hope Through Research", published by the NINDS.
Research into treating the condition with Mirror Visual Feedback is being undertaken at the Royal National Hospital for Rheumatic Disease in Bath. Patients are taught how to desensitize in the most effective way, then progress to using mirrors to rewrite the faulty signals in the brain that appear responsible for this condition.
The Netherlands currently has the most comprehensive program of research into CRPS, as part of a multi-million Euro initiative called TREND.[84] German and Australian research teams are also pursuing better understanding and treatments for CRPS.
Other animals
CRPS has also been described in animals.[85]
References
- ↑ Schwartzman RJ, Erwin KL, Alexander GM (May 2009). "The natural history of complex regional pain syndrome". The Clinical Journal of Pain 25 (4): 273–80. doi:10.1097/AJP.0b013e31818ecea5. PMID 19590474.
- ↑ Schwartzman, Robert J. (2012). "Systemic Complications of Complex Regional Pain Syndrome". Neuroscience & Medicine 3 (3): 225–42. doi:10.4236/nm.2012.33027.
- ↑ Jänig W, Baron R (June 2002). "Complex regional pain syndrome is a disease of the central nervous system". Clinical Autonomic Research 12 (3): 150–64. doi:10.1007/s10286-002-0022-1. PMID 12269546.
- ↑ Lohnberg JA, Altmaier EM (June 2013). "A review of psychosocial factors in complex regional pain syndrome". Journal of Clinical Psychology in Medical Settings 20 (2): 247–54. doi:10.1007/s10880-012-9322-3. PMID 22961122.
- ↑ "Neuropathic pain". Merck Manual for Healthcare Professionals.
- ↑ American RSD Hope Marks and Harrison http://www.rsdhope.org/mcgill-pain-index---where-is-crps-pain-ranked.html
- 1 2 Marinus J, Moseley GL, Birklein F; et al. (July 2011). "Clinical features and pathophysiology of complex regional pain syndrome". Lancet Neurology 10 (7): 637–48. doi:10.1016/S1474-4422(11)70106-5. PMID 21683929.
- ↑ "Welcome to Map of Medicine Healthguides". Healthguides.mapofmedicine.com. Retrieved 2013-12-23.
- 1 2 Eberle T, Doganci B, Krämer HH; et al. (February 2009). "Warm and cold complex regional pain syndromes: differences beyond skin temperature?". Neurology 72 (6): 505–12. doi:10.1212/01.wnl.0000341930.35494.66. PMID 19204260.
- ↑ Vaneker M, Wilder-Smith OH, Schrombges P, de Man-Hermsen I, Oerlemans HM (May 2005). "Patients initially diagnosed as "warm" or "cold" CRPS 1 show differences in central sensory processing some eight years after diagnosis: a quantitative sensory testing study". Pain 115 (1-2): 204–11. doi:10.1016/j.pain.2005.02.031. PMID 15836983.
- 1 2 3 Veldman PH, Reynen HM, Arntz IE, Goris RJ (October 1993). "Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients". Lancet 342 (8878): 1012–6. doi:10.1016/0140-6736(93)92877-V. PMID 8105263.
- ↑ Yu D (August 2004). "Shoulder pain in hemiplegia". Physical Medicine and Rehabilitation Clinics of North America 15 (3): vi–vii, 683–97. doi:10.1016/S1047-9651(03)00130-X. PMID 15219895.
- ↑ Pawelka S, Fialka V, Ernst E (January 1993). "Reflex sympathetic dystrophy and cigarette smoking". The Journal of Hand Surgery 18 (1): 168–9. doi:10.1016/0363-5023(93)90273-6. PMID 8423309.
- ↑ Maihöfner C, Forster C, Birklein F, Neundörfer B, Handwerker HO (March 2005). "Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study". Pain 114 (1-2): 93–103. doi:10.1016/j.pain.2004.12.001. PMID 15733635.
- 1 2 DR WILL HOWARD FFPMANZCA, FFANZCA, DIP MED (PAIN MANAGEMENT) http://www.qld.anzca.edu.au/anzca/resources/college-publications/pdfs/ANZCA%20Blue%20Book%202011%20P9.pdf#page=10[]
- ↑ "Pain". Courses.washington.edu. Retrieved 2013-12-23.
- 1 2 Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE (September 2004). "Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome". Pain Medicine 5 (3): 263–75. doi:10.1111/j.1526-4637.2004.04043.x. PMID 15367304.
- ↑ Kiefer RT, Rohr P, Ploppa A; et al. (2008). "A pilot open-label study of the efficacy of subanesthetic isomeric S(+)-ketamine in refractory CRPS patients". Pain Med 9 (1): 44–54. doi:10.1111/j.1526-4637.2006.00223.x. PMID 18254766.
- ↑ Pöyhiä R, Vainio A (January 2006). "Topically administered ketamine reduces capsaicin-evoked mechanical hyperalgesia". The Clinical Journal of Pain 22 (1): 32–6. doi:10.1097/01.ajp.0000149800.39240.95. PMID 16340591.
- ↑ Watkins LR, Maier SF (February 2005). "Immune regulation of central nervous system functions: from sickness responses to pathological pain". Journal of Internal Medicine 257 (2): 139–55. doi:10.1111/j.1365-2796.2004.01443.x. PMID 15656873.
- ↑ Koffler SP, Hampstead BM, Irani F; et al. (August 2007). "The neurocognitive effects of 5 day anesthetic ketamine for the treatment of refractory complex regional pain syndrome". Archives of Clinical Neuropsychology 22 (6): 719–29. doi:10.1016/j.acn.2007.05.005. PMID 17611073.
- ↑ Birklein F (February 2005). "Complex regional pain syndrome". Journal of Neurology 252 (2): 131–8. doi:10.1007/s00415-005-0737-8. PMID 15729516.
- ↑ Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW (July 2007). "Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study". The Journal of Bone and Joint Surgery. American Volume 89 (7): 1424–31. doi:10.2106/JBJS.F.01147. PMID 17606778.
- ↑ Quisel A, Gill JM, Witherell P (2005). "Complex regional pain syndrome underdiagnosed". J Fam Pract 54 (6): 524–32. PMID 15939004.
- ↑ Birklein F, Künzel W, Sieweke N (August 2001). "Despite clinical similarities there are significant differences between acute limb trauma and complex regional pain syndrome I (CRPS I)". Pain 93 (2): 165–71. doi:10.1016/s0304-3959(01)00309-8. PMID 11427328.
- ↑ Wasner G, Schattschneider J, Baron R (July 2002). "Skin temperature side differences--a diagnostic tool for CRPS?". Pain 98 (1-2): 19–26. doi:10.1016/s0304-3959(01)00470-5. PMID 12098613.
- ↑ Gulevich SJ, Conwell TD, Lane J; et al. (March 1997). "Stress infrared telethermography is useful in the diagnosis of complex regional pain syndrome, type I (formerly reflex sympathetic dystrophy)". Clin J Pain 13 (1): 50–9. doi:10.1097/00002508-199703000-00008. PMID 9084952.
- ↑ "Reflex Sympathetic Dystrophy Clinical Practice Guidelines". Rsdfoundation.org. 2003-01-01. Retrieved 2013-12-23.
- ↑ Meena, S; Sharma, P; Gangary, SK; Chowdhury, B (9 December 2014). "Role of vitamin C in prevention of complex regional pain syndrome after distal radius fractures: a meta-analysis.". European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. doi:10.1007/s00590-014-1573-2. PMID 25488053.
- ↑ Lee BH, Scharff L, Sethna NF; et al. (July 2002). "Physical therapy and cognitive-behavioral treatment for complex regional pain syndromes". The Journal of Pediatrics 141 (1): 135–40. doi:10.1067/mpd.2002.124380. PMID 12091866.
- 1 2 Daly AE, Bialocerkowski AE (April 2009). "Does evidence support physiotherapy management of adult Complex Regional Pain Syndrome Type One? A systematic review". European Journal of Pain 13 (4): 339–53. doi:10.1016/j.ejpain.2008.05.003. PMID 18619873.
- 1 2 Shah A, Kirchner JS (June 2011). "Complex regional pain syndrome". Foot and Ankle Clinics 16 (2): 351–66. doi:10.1016/j.fcl.2011.03.001. PMID 21600455.
- 1 2 van de Meent H, Oerlemans M, Bruggeman A; et al. (June 2011). "Safety of 'pain exposure' physical therapy in patients with complex regional pain syndrome type 1". Pain 152 (6): 1431–8. doi:10.1016/j.pain.2011.02.032. PMID 21474244.
- ↑ http://www.chop.edu/service/amplified-musculoskeletal-pain-syndrome/about-amps/amps-treatment.html[]
- ↑ Sherry DD, Wallace CA, Kelley C, Kidder M, Sapp L (September 1999). "Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy". The Clinical Journal of Pain 15 (3): 218–23. doi:10.1097/00002508-199909000-00009. PMID 10524475.
- ↑ McCabe CS, Haigh RC, Ring EF, Halligan PW, Wall PD, Blake DR (January 2003). "A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1)". Rheumatology (Oxford) 42 (1): 97–101. doi:10.1093/rheumatology/keg041. PMID 12509620.
- 1 2 Moseley, G; Zalucki, N; Wiech, K (2008). "Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain". Pain 137 (3): 600–8. doi:10.1016/j.pain.2007.10.021. PMID 18054437.
- ↑ Swart CM, Stins JF, Beek PJ (October 2009). "Cortical changes in complex regional pain syndrome (CRPS)". Eur J Pain 13 (9): 902–7. doi:10.1016/j.ejpain.2008.11.010. PMID 19101181.
- ↑ Archived November 2, 2013 at the Wayback Machine
- ↑ noi — graded motor imagery
- ↑ Moseley GL (March 2004). "Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial". Pain 108 (1-2): 192–8. doi:10.1016/j.pain.2004.01.006. PMID 15109523.
- ↑ Moseley GL (March 2005). "Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial". Pain 114 (1-2): 54–61. doi:10.1016/j.pain.2004.11.024. PMID 15733631.
- ↑ "Graded motor imagery for chronic pain". Bodyinmind.com.au. 2009-10-07. Archived from the original on March 23, 2010. Retrieved 2010-04-10.
- ↑ "Graded Motor Imagery". Graded Motor Imagery. Retrieved 2013-12-23.
- ↑ Flor, H; Denke, C; Schaefer, M; Grüsser, S (2001). "Effect of sensory discrimination training on cortical reorganisation and phantom limb pain.". Lancet 357 (9270): 1763–4. doi:10.1016/S0140-6736(00)04890-X. PMID 11403816.
- ↑ "Training the Brain 3 – Brain changes, S1 reorganisation". Bodyinmind.com.au. 2009-11-25. Retrieved 2010-04-10.
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3661907/
- ↑ Azari, P; Lindsay, DR; Briones, D; Clarke, C; Buchheit, T; Pyati, S (1 March 2012). "Efficacy and safety of ketamine in patients with complex regional pain syndrome: a systematic review.". CNS Drugs 26 (3): 215–28. doi:10.2165/11595200-000000000-00000. PMID 22136149.
- ↑ Schwartzman, RJ; Alexander, GM; Grothusen, JR (May 2011). "The use of ketamine in complex regional pain syndrome: possible mechanisms.". Expert Review of Neurotherapeutics 11 (5): 719–34. doi:10.1586/ern.11.31. PMID 21539489.
- 1 2 Niesters, M; Martini, C; Dahan, A (February 2014). "Ketamine for chronic pain: risks and benefits.". British Journal of Clinical Pharmacology 77 (2): 357–67. doi:10.1111/bcp.12094. PMID 23432384.
- ↑ Pontell D (July 2008). "A clinical approach to complex regional pain syndrome". Clinics in Podiatric Medicine and Surgery 25 (3): 361–80; vi. doi:10.1016/j.cpm.2008.02.011. PMID 18486850.
- ↑ Kubalek I, Fain O, Paries J, Kettaneh A, Thomas M (December 2001). "Treatment of reflex sympathetic dystrophy with pamidronate: 29 cases". Rheumatology 40 (12): 1394–7. doi:10.1093/rheumatology/40.12.1394. PMID 11752511.
- ↑ Varenna M, Zucchi F, Ghiringhelli D; et al. (June 2000). "Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study". The Journal of Rheumatology 27 (6): 1477–83. PMID 10852274.
- 1 2 3 Varenna M, Adami S, Rossini M; et al. (March 2013). "Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study". Rheumatology 52 (3): 534–42. doi:10.1093/rheumatology/kes312. PMID 23204550.
- ↑ Robinson JN, Sandom J, Chapman PT (September 2004). "Efficacy of pamidronate in complex regional pain syndrome type I". Pain Medicine 5 (3): 276–80. doi:10.1111/j.1526-4637.2004.04038.x. PMID 15367305.
- ↑ Brunner F, Schmid A, Kissling R, Held U, Bachmann LM (January 2009). "Biphosphonates for the therapy of complex regional pain syndrome I--systematic review". European Journal of Pain 13 (1): 17–21. doi:10.1016/j.ejpain.2008.03.005. PMID 18440845.
- ↑ Zuurmond WW, Langendijk PN, Bezemer PD, Brink HE, de Lange JJ, van loenen AC (March 1996). "Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream". Acta Anaesthesiologica Scandinavica 40 (3): 364–7. doi:10.1111/j.1399-6576.1996.tb04446.x. PMID 8721469.
- 1 2 Kopsky DJ, Keppel Hesselink JM (2011). "Multimodal Stepped Care Approach Involving Topical Analgesics for Severe Intractable Neuropathic Pain in CRPS Type 1: A Case Report". Case Reports in Medicine 2011: 319750. doi:10.1155/2011/319750. PMC 3199095. PMID 22028723.
- ↑ Keppel Hesselink JM, Kopsky DJ (2013). "Treatment of chronic regional pain syndrome type 1 with palmitoylethanolamide and topical ketamine cream: modulation of nonneuronal cells". Journal of Pain Research 6: 239–45. doi:10.2147/JPR.S42417. PMC 3643547. PMID 23658493.
- ↑ Kharkar S, Ambady P, Venkatesh Y, Schwartzman RJ (2011). "Intramuscular botulinum toxin in complex regional pain syndrome: case series and literature review". Pain Physician 14 (5): 419–24. PMID 21927045.
- ↑ Taylor RS, Van Buyten JP, Buchser E (February 2006). "Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors". European Journal of Pain 10 (2): 91–101. doi:10.1016/j.ejpain.2005.02.004. PMID 16310712.
- ↑ Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M (February 2008). "Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial". J. Neurosurg. 108 (2): 292–8. doi:10.3171/JNS/2008/108/2/0292. PMID 18240925.
- ↑ Stanton-Hicks M, Baron R, Boas R; et al. (June 1998). "Complex Regional Pain Syndromes: guidelines for therapy". The Clinical Journal of Pain 14 (2): 155–66. doi:10.1097/00002508-199806000-00012. PMID 9647459.
- ↑ Bodde MI, Dijkstra PU, den Dunnen WF, Geertzen JH (October 2011). "Therapy-resistant complex regional pain syndrome type I: to amputate or not?". The Journal of Bone and Joint Surgery. American Volume 93 (19): 1799–805. doi:10.2106/JBJS.J.01329. PMID 22005865.
- ↑ Grunert BK, Devine CA, Sanger JR, Matloub HS, Green D (July 1990). "Thermal self-regulation for pain control in reflex sympathetic dystrophy syndrome". The Journal of Hand Surgery 15 (4): 615–8. doi:10.1016/S0363-5023(09)90024-7. PMID 2199569.
- ↑ The Psychologist's Role in the Chronic Pain of Reflex Sympathetic Dystrophy. Rosemarie Scolaro Moser, Ph.D. Printed in New Jersey Psychologist, Spring 1999, pp. 24–25.
- ↑ Gainer MJ (1992). "Hypnotherapy for reflex sympathetic dystrophy". Am J Clin Hypn 34 (4): 227–32. PMID 1349789.
- ↑ "RSDSA :: Reflex Sympathetic Dystrophy Syndrome Association". Rsds.org. 2010-01-21. Retrieved 2010-04-10.
- ↑ Mitchell, S.W. (1872). Injuries of Nerves and their Consequences. Philadelphia: JB Lippincott.
- ↑ Richards RL (January 1967). "The term 'causalgia'". Medical History 11 (1): 97–9. doi:10.1017/s0025727300011789. PMC 1033672. PMID 5341040.
- ↑ Spicher, C.J. (2006). Handbook for Somatosensory Rehabilitation. Montpellier, Paris: Sauramps Medical.
- ↑ EVANS JA (March 1947). "Reflex sympathetic dystrophy; report on 57 cases". Annals of Internal Medicine 26 (3): 417–26. doi:10.7326/0003-4819-26-3-417. PMID 20288177.
- ↑ Noordenbos, W. (1959). PAIN Problems pertaining to the transmission of nerve impulses which give rise to pain. Amsterdam: Elsevier.
- ↑ Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P (October 1995). "Reflex sympathetic dystrophy: changing concepts and taxonomy". Pain 63 (1): 127–33. doi:10.1016/0304-3959(95)00110-E. PMID 8577483.
- ↑ Paula Abdul http://www.rarediseaseawareness.com/quality-of-life/paula-abdul-putting-the-spotlight-on-rare-disease
- ↑ LA Times: Jill Kinmont Boothe is still going strong http://articles.latimes.com/2011/may/22/sports/la-sp-crowe-20110523
- ↑ Gemma Collis http://www.paralympics.org.uk/gb/athletes/gemma-collis[]
- ↑ Shin Dong-Wook http://www.allkpop.com/2013/01/actor-shin-dong-wook-suffering-from-rare-disease
- ↑ Forest Tennant, MD, DrPH (July–August 2007). "Howard Hughes & Pseudoaddiction" (PDF). Practical Pain Management (PPM Communications, Inc.) 6 (7): 12–29. Archived from the original (PDF) on September 25, 2007. Retrieved January 7, 2011.
- ↑ Barby Ingle http://www.painpathways.org/barby-ingle-on-crps
- ↑ Rachel Morris http://www.paralympics.org.uk/gb/athletes/rachel-morris
- ↑ Cynthia Toussaint http://www.lifescript.com/health/centers/pain/articles/how_a_hamstring_tear_derailed_a_dancers_life.aspx
- ↑ BBC http://www.bbc.co.uk/sport/disability-sport/27064013
- ↑ TREND homepage.
- ↑ Bergadano A, Moens Y, Schatzmann U (May 2006). "Continuous extradural analgesia in a cow with complex regional pain syndrome". Veterinary Anaesthesia and Analgesia 33 (3): 189–92. doi:10.1111/j.1467-2995.2005.00245.x. PMID 16634945.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||