Cation-anion radius ratio
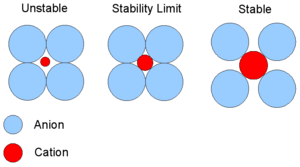
In condensed matter physics and inorganic chemistry the cation-anion radius ratio is the ratio of the ionic radius of the cation to the ionic radius of the anion in a cation-anion compound. This is simply given by 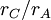 .
.
According to Pauling's rules for crystal structures, the allowed size of the cation for a given structure is determined by the critical radius ratio.[1] If the cation is too small, then it will attract the anions into each other and they will collide hence the compound will be unstable due to anion-anion repulsion; this occurs when the radius ratio drops below 0.155.
At the stability limit the cation is touching all the anions and the anions are just touching at their edges (radius ratio = 0.155). Beyond this stability limit (radius ratio < 0.155) the compound may be stable.
The table below gives the relation between radius ratio and coordination number, which may be obtained from a simple geometrical proof.[2]
| Radius Ratio | Coordination number | Type of void | Example |
|---|---|---|---|
| < 0.155 | 2 | Linear | |
| 0.155 - 0.225 | 3 | Triangular Planar | B2CO3 |
| 0.225 - 0.414 | 4 | Tetrahedral | ZnS, CuCl |
| 0.414 - 0.732 | 6 | Octahedral | NaCl, MgO |
| 0.732 - 1.000 | 8 | Cubic | CsCl, NH4Br |
See also
References
- ↑ Pauling, Linus (1960). The nature of the chemical bond and the structure of molecules and crystals; an introduction to modern structural chemistry (3rd ed.). Ithaca (NY): Cornell University Press. p. 544-547. ISBN 0-8014-0333-2.
- ↑ Toofan J. (1994) J. Chem. Educ. 71 (9), 147 (and Erratum p.749) A Simple Expression between Critical Radius Ratio and Coordination Numbers