Catellani Reaction
The Catellani reaction was devised by Marta Catellani. It is Norbornene-mediated Ortho C-H functionalization consisting series of reaction.[1] Norbornene acts as a catalyst in this reaction.[2]
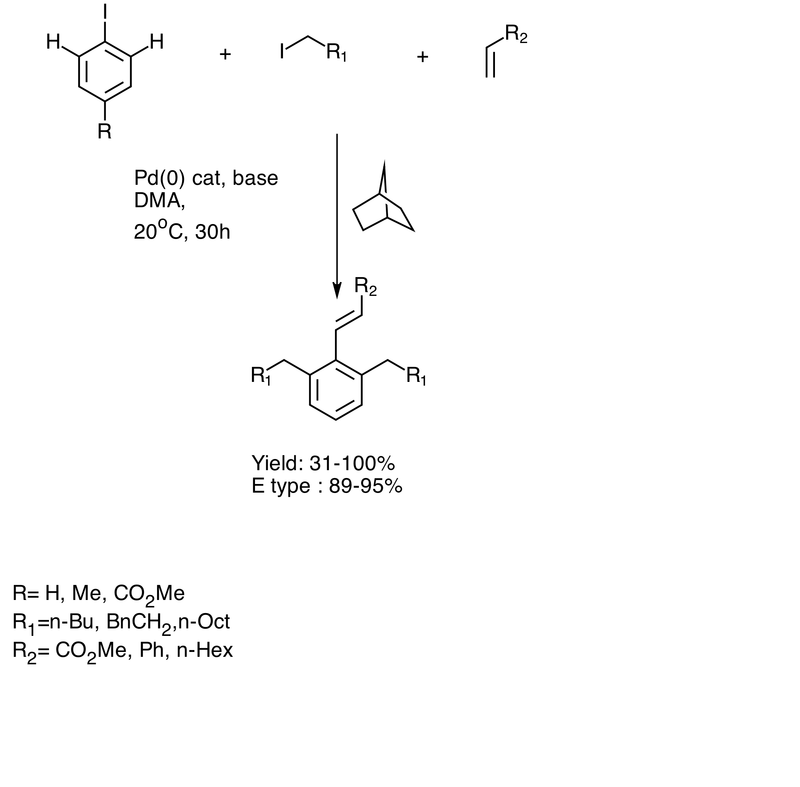
Reaction mechanism
The Catellani reaction is catalyzed by norbornene and palladium. The key step is the formation of a palladacycle by electrophilic metalation at the ortho position followed by deprotonation and rearomatization. The unique structure of the norbornene allows this step to occur because there is no possibility of beta hydride elimination with the bridgehead hydrogen.[3] Termination can occur by any of a number of traditional palladium-catalyzed coupling reactions.
Rhodium can also be used as a catalyst along with norbornene instead of Palladium.[4]
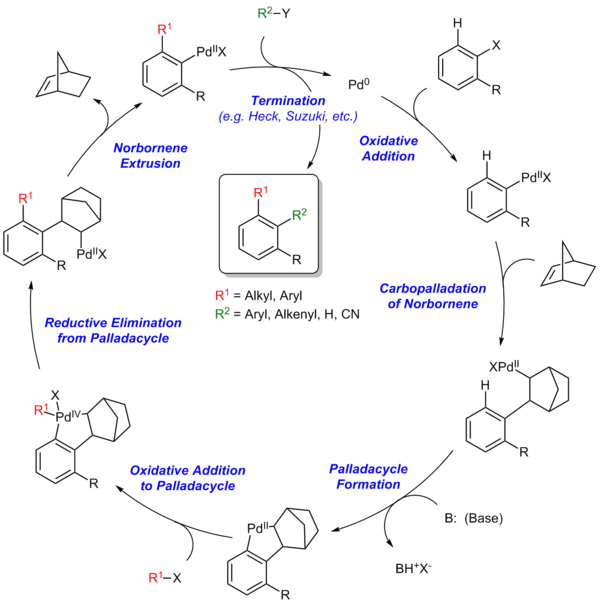
Steps of the Catellani reaction:
- Oxidative addition
- Carbopalladation of norbornene
- Palladacycle formation
- Oxidative addition to palladacycle
- Reductive elimination from palladacycle
- Norbornene extrusion
- Termination via Heck reaction, Suzuki reaction, etc.
Uses
Catellani reaction is used for polyfunctionalization of aromatic molecule. It has been used as a key step for synthesis of novel lignan (+)- linoxepin.[6] It can also be used for synthesis of Rhazinal.[7]
References
- ↑ Catellani; et al. (1997). "Regioselektive Synthese o,o′-disubstituierter Vinylarene über einen komplexen Katalysecyclus". Angewandte Chemie 109 (1-2). doi:10.1002/ange.19971090146. Retrieved 26 December 2014.
- ↑ Catellani; et al. (1997). "Regioselektive Synthese o,o′-disubstituierter Vinylarene über einen komplexen Katalysecyclus". Angewandte Chemie 109 (1-2). doi:10.1002/ange.19971090146. Retrieved 26 December 2014.
- ↑ Martins; et al. (2010). "Synthesis in the Key of Catellani: Norbornene-Mediated ortho C–H Functionalization". Top Curr Chem 292. doi:10.1007/128_2009_13. Retrieved 25 November 2015.
- ↑ Wu; et al. (2013). "An efficient method for the Heck–Catellani reaction of aryl halides". Chem. Commun. 49. doi:10.1039/C3CC46381H.
- ↑ Martins; et al. (2010). "Synthesis in the Key of Catellani: Norbornene-Mediated ortho C–H Functionalization". Top Curr Chem 292. doi:10.1007/128_2009_13. Retrieved 25 November 2015.
- ↑ Weinstabl; et al. (16 Apr 2013). "Total Synthesis of (+)-Linoxepin by Utilizing the Catellani Reaction". Angewandte Chemie International Edition 52 (20). doi:10.1002/anie.201302327. Retrieved 26 December 2014.
- ↑ Sui; et al. (June 2013). "Pd-Catalyzed Chemoselective Catellani Ortho-Arylation of Iodopyrroles: Rapid Total Synthesis of Rhazinal". J. Am. Chem. Soc 135 (25): 9318–9321. doi:10.1021/ja404494u. Retrieved 26 December 2014.