Carotene


The term carotene (also carotin, from the Latin carota, "carrot"[1][2]) is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals (with the sole known exception of some aphids and spider mites which acquired the synthetic genes from fungi).[3] Carotenes are photosynthetic pigments important for photosynthesis. Carotenes contain no oxygen. They absorb ultraviolet, violet, and blue light and emit orange or red light, and (in low concentrations) yellow light.
Carotenes are responsible for the orange colour of the carrot, for which this class of chemicals is named, and for the colours of many other fruits and vegetables (for example, sweet potatoes, chanterelle and orange cantaloupe melon). Carotenes are also responsible for the orange (but not all of the yellow) colours in dry foliage. They also (in lower concentrations) impart the yellow coloration to milk-fat and butter. Omnivorous animal species which are relatively poor converters of coloured dietary carotenoids to colourless retinoids have yellowed-coloured body fat, as a result of the carotenoid retention from the vegetable portion of their diet. The typical yellow-coloured fat of humans and chickens is a result of fat storage of carotenes from their diets.
Carotenes contribute to photosynthesis by transmitting the light energy they absorb to chlorophyll. They also protect plant tissues by helping to absorb the energy from singlet oxygen, an excited form of the oxygen molecule O2 which is formed during photosynthesis.
β-Carotene is composed of two retinyl groups, and is broken down in the mucosa of the human small intestine by β-carotene 15,15'-monooxygenase to retinal, a form of vitamin A. β-Carotene can be stored in the liver and body fat and converted to retinal as needed, thus making it a form of vitamin A for humans and some other mammals. The carotenes α-carotene and γ-carotene, due to their single retinyl group (β-ionone ring), also have some vitamin A activity (though less than β-carotene), as does the xanthophyll carotenoid β-cryptoxanthin. All other carotenoids, including lycopene, have no beta-ring and thus no vitamin A activity (although they may have antioxidant activity and thus biological activity in other ways).
Animal species differ greatly in their ability to convert retinyl (beta-ionone) containing carotenoids to retinals. Carnivores in general are poor converters of dietary ionone-containing carotenoids. Pure carnivores such as ferrets lack β-carotene 15,15'-monooxygenase and cannot convert any carotenoids to retinals at all (resulting in carotenes not being a form of vitamin A for this species); while cats can convert a trace of β-carotene to retinol, although the amount is totally insufficient for meeting their daily retinol needs.[4]
Molecular structure
Chemically, carotenes are polyunsaturated hydrocarbons containing 40 carbon atoms per molecule, variable numbers of hydrogen atoms, and no other elements. Some carotenes are terminated by hydrocarbon rings, on one or both ends of the molecule. All are coloured to the human eye, due to extensive systems of conjugated double bonds. Structurally carotenes are tetraterpenes, meaning that they are synthesized biochemically from four 10-carbon terpene units, which in turn are formed from eight 5-carbon isoprene units.
Carotenes are found in plants in two primary forms designated by characters from the Greek alphabet: alpha-carotene (α-carotene) and beta-carotene (β-carotene). Gamma-, delta-, epsilon-, and zeta-carotene (γ, δ, ε, and ζ-carotene) also exist. Since they are hydrocarbons, and therefore contain no oxygen, carotenes are fat-soluble and insoluble in water (in contrast with other carotenoids, the xanthophylls, which contain oxygen and thus are less chemically hydrophobic).
Dietary sources
The following foods are particularly rich in carotenes[5] (also see Vitamin A article for amounts):
- sweet potatoes[6]
- carrots[6][7]
- wolfberries (goji)[8]
- cantaloupe melon[9]
- mangoes[10]
- apricots[10]
- Persimmon[7]
- spinach[6][7]
- kale[6][7]
- chard[7]
- turnip greens[6][7]
- dandelion greens[7]
- beet greens[7]
- mustard greens[7][11]
- collard greens[6]
- watercress[7]
- cilantro (coriander)[6]
- fresh thyme[6]
- broccoli[7]
- parsley[7]
- romaine lettuce[6]
- ivy gourd[12]
- rose hips[13]
- winter squash[6][7]
- pumpkin[7]
- cassava[14]
Absorption from these foods is enhanced if eaten with fats, as carotenes are fat soluble, and if the food is cooked for a few minutes until the plant cell wall splits and the colour is released into any liquid. 6 μg of dietary β-carotene supplies the equivalent of 1 μg of retinol, or 1 RE (Retinol Equivalent). This is equivalent to 3⅓ IU of vitamin A.
The multiple forms
The two primary isomers of carotene, α-carotene and β-carotene, differ in the position of a double bond (and thus a hydrogen) in the cyclic group at one end (the right end in the diagram at right).
β-Carotene is the more common form and can be found in yellow, orange, and green leafy fruits and vegetables. As a rule of thumb, the greater the intensity of the orange colour of the fruit or vegetable, the more β-carotene it contains.
Carotene protects plant cells against the destructive effects of ultraviolet light. β-Carotene is an antioxidant.
β-Carotene and cancer
It has been shown in trials that the ingestion of β-carotene supplements at about 30 mg/day (10 times the Reference Daily Intake) increases the rate of lung and prostate cancer development in smokers and people with a history of asbestos exposure.
An article on the American Cancer Society says that The Cancer Research Campaign has called for warning labels on β-carotene supplements to caution smokers that such supplements may increase the risk of lung cancer.[15]
The New England Journal of Medicine published an article[16] in 1994 about a trial which examined the relationship between daily supplementation of β-carotene and vitamin E (α-tocopherol) and the incidence of lung cancer. The study was done using supplements and researchers were aware of the epidemiological correlation between carotenoid-rich fruits and vegetables and lower lung cancer rates. The research concluded that no reduction in lung cancer was found in the participants using these supplements, and furthermore, these supplements may, in fact, have harmful effects.
The Journal of the National Cancer Institute and The New England Journal of Medicine published articles in 1996[17][18] about a trial that was conducted to determine if vitamin A (in the form of retinyl palmitate) and β-carotene had any beneficial effects to prevent cancer. The results indicated an increased risk of lung cancer for the participants who consumed the β-carotene supplement and who had lung irritation from smoking or asbestos exposure, causing the trial to be stopped early.[18]
A review of all randomized controlled trials in the scientific literature by the Cochrane Collaboration published in JAMA in 2007 found that synthetic β-carotene increased mortality by something between 1 and 8% (Relative Risk 1.05, 95% confidence interval 1.01–1.08).[19] However, this meta-analysis included two large studies of smokers, so it is not clear that the results apply to the general population.[20] The review only studied the influence of synthetic antioxidants and the results should not be translated to potential effects of fruits and vegetables.
β-Carotene and cognition
A recent report demonstrated that 50 mg of β-carotene every other day prevented cognitive decline in a study of over 4000 physicians at a mean treatment duration of 18 years.[21]
β-Carotene and photosensitivity
Oral β-carotene is prescribed to people suffering from erythropoietic protoporphyria. It provides them some relief from photosensitivity.
β-Carotene and nanotechnology
β-Carotene and lycopene molecules can be encapsulated into carbon nanotubes enhancing the optical properties of carbon nanotubes.[22] Efficient energy transfer occurs between the encapsulated dye and nanotube — light is absorbed by the dye and without significant loss is transferred to the single wall carbon nanotube (SWCNT). Encapsulation increases chemical and thermal stability of carotene molecules; it also allows their isolation and individual characterization.[23]
Carotenemia
Carotenemia or hypercarotenemia is excess carotene, but unlike excess vitamin A, carotene is non-toxic. Although hypercarotenemia is not particularly dangerous, it can lead to an oranging of the skin (carotenodermia), but not the conjunctiva of eyes (thus easily distinguishing it visually from jaundice). It is most commonly associated with consumption of an abundance of carrots, but it also can be a medical sign of more dangerous conditions.
Production

Most of the world's synthetic supply of carotene comes from a manufacturing complex located in Freeport, Texas and owned by DSM. The other major supplier BASF also uses a chemical process to produce β-carotene. Together these suppliers account for about 85% of the β-carotene on the market. In Spain Vitatene produces natural β-carotene from fungus Blakeslea trispora, as does DSM but at much lower amount when compared to its synthetic β-carotene operation. In Australia, organic β-carotene is produced by Aquacarotene Limited from dried marine algae Dunaliella salina grown in harvesting ponds situated in Karratha, Western Australia. BASF Australia is also producing β-carotene from microalgae grown in two sites in Australia that are the world’s largest algae farms. In Portugal, the industrial biotechnology company Biotrend is producing natural all-trans-β-carotene from a non genetically modified bacteria of the Sphingomonas genus isolated from soil.
Carotenes are also found in palm oil, corn, and in the milk of dairy cows, causing cow's milk to be light yellow, depending on the feed of the cattle, and the amount of fat in the milk (high-fat milks, such as those produced by Guernsey cows, tend to be yellower because their fat content causes them to contain more carotene).
Carotenes are also found in some species of termites, where they apparently have been picked up from the diet of the insects.
Total synthesis
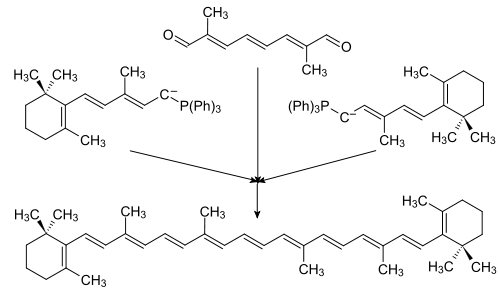
There are currently two commonly used methods of total synthesis of β-carotene. The first was developed by the Badische Anilin- & Soda-Fabrik (BASF) and is based on the Wittig reaction with Wittig himself as patent holder:[24][25]
The second is a Grignard reaction, elaborated by Hoffman-La Roche from the original synthesis of Inhoffen et al. They are both symmetrical; the BASF synthesis is C20 + C20, and the Hoffman-La Roche synthesis is C19 + C2 + C19.
Nomenclature
Carotenes are carotenoids containing no oxygen. Carotenoids containing some oxygen are known as xanthophylls.
The two ends of the β-carotene molecule are structurally identical, and are called β-rings. Specifically, the group of nine carbon atoms at each end form a β-ring.
The α-carotene molecule has a β-ring at one end; the other end is called an ε-ring. There is no such thing as an "α-ring".
These and similar names for the ends of the carotenoid molecules form the basis of a systematic naming scheme, according to which:
- α-carotene is β,ε-carotene;
- β-carotene is β,β-carotene;
- γ-carotene (with one β ring and one uncyclized end that is labelled psi) is β,ψ-carotene;
- δ-carotene (with one ε ring and one uncyclized end) is ε,ψ-carotene;
- ε-carotene is ε,ε-carotene
- lycopene is ψ,ψ-carotene
ζ-Carotene is the biosynthetic precursor of neurosporene, which is the precursor of lycopene, which, in turn, is the precursor of the carotenes α through ε.
Food additive
Carotene is also used as a substance to colour products such as juice, cakes, desserts, butter and margarine. It is approved for use as a food additive in the EU (listed as additive E160a)[26] Australia and New Zealand (listed as 160a)[27] and the US.[28]
See also
References
- ↑ Mosby’s Medical, Nursing and Allied Health Dictionary, Fourth Edition, Mosby-Year Book 1994, p. 273
- ↑ "carotene". Online Etymology Dictionary.
- ↑ Boran Altincicek, Jennifer L. Kovacs & Nicole M. Gerardo (2011). "Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae". Biology Letters 8 (2): 253–257. doi:10.1098/rsbl.2011.0704. PMC 3297373. PMID 21920958.
- ↑ Green AS, Tang G, Lango J, Klasing KC, Fascetti AJ (2011). "Domestic cats convert ((2) H(8))-β-carotene to ((2) H(4))-retinol following a single oral dose". Journal of Animal Physiology and Animal Nutrition 96 (4): 681–92. doi:10.1111/j.1439-0396.2011.01196.x. PMID 21797934.
- ↑ Linus Pauling Institute (June 2009). "Micronutrient Information Center: Carotenoids". Oregon State University. Retrieved February 15, 2010.
- 1 2 3 4 5 6 7 8 9 10 What can foods rich in beta-carotene do for you?. Whfoods.org (2004-02-26). Retrieved on 2013-03-04.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Pitchford, Paul (2002). Healing with Whole Foods: Asian Traditions and Modern Nutrition. North Atlantic Books. ISBN 1-55643-471-5.
- ↑ Ajila CM, Prasada Rao UJ (2008). "Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS". J Pharm Biomed Anal 47 (4–5): 812–8. doi:10.1016/j.jpba.2008.04.001. PMID 18486400.
- ↑ Olson, James Allen; Rock, Cheryl L.; Ross, A. Catharine and Underwood, Barbara A. (2006-04-23). "Dietary Supplement Fact Sheet: Vitamin A". NIH Office of Dietary Supplements website. United States Department of Agriculture. Retrieved 2007-10-26.
- 1 2 World's Healthiest Foods: Carotenoids. Whfoods.org. Retrieved on 2013-03-04.
- ↑ Mustard greens. WHFoods. Retrieved on 2013-03-04.
- ↑ Simopoulos, Artemis P. and Gopalan, C., ed. (2003). Plants in Human Health and Nutrition Policy. Karger Publishers. ISBN 3-8055-7554-8.
- ↑ Valadon, L. R. G. and Mummery, Rosemary S. "Changes in Carotenoid Composition of Certain Roses with Age". Annals of Botany 33 (4): 671–677.
- ↑ Adewusi, Steve R A; Bradbury, J Howard (1993). "Carotenoids in cassava: Comparison of open-column and HPLC methods of analysis". Journal of the Science of Food and Agriculture 62 (4): 375. doi:10.1002/jsfa.2740620411.
- ↑ "British Cancer Organization Calls for Warning Labels on Beta-Carotene". 2000-07-31. Archived from the original on 2006-12-04. Retrieved 2007-03-15.
- ↑ The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group (1994). "The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers". N Engl J Med. 330 (15): 1029–35. doi:10.1056/NEJM199404143301501. PMID 8127329.
- ↑ Omenn GS, Goodman GE, Thornquist MD; et al. (1996). "Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial". J Natl Cancer Inst. 88 (21): 1550–9. doi:10.1093/jnci/88.21.1550. PMID 8901853.
- 1 2 Omenn GS, Goodman GE, Thornquist MD; et al. (1996). "Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease". N Engl J Med. 334 (18): 1150–5. doi:10.1056/NEJM199605023341802. PMID 8602180.
- ↑ Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007). "Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis". JAMA 297 (8): 842–57. doi:10.1001/jama.297.8.842. PMID 17327526.
- ↑ See the letter to JAMA by Philip Taylor and Sanford Dawsey and the reply by the authors of the original paper.
- ↑ Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM (Nov 2007). "A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II". Arch Intern Med. 167 (20): 2184–90. doi:10.1001/archinte.167.20.2184. PMID 17998490.
- ↑ Yanagi, Kazuhiro; Iakoubovskii, Konstantin; Kazaoui, Said; Minami, Nobutsugu; Maniwa, Yutaka; Miyata, Yasumitsu; Kataura, Hiromichi (2006). "Light-Harvesting Function of β-Carotene Inside Carbon Nanotubes" (PDF). Phys. Rev. B 74 (15): 155420. Bibcode:2006PhRvB..74o5420Y. doi:10.1103/PhysRevB.74.155420.
- ↑ Saito, Yuika; Yanagi, Kazuhiro; Hayazawa, Norihiko; Ishitobi, Hidekazu; Ono, Atsushi; Kataura, Hiromichi; Kawata, Satoshi (2006). "Vibrational Analysis of Organic Molecules Encapsulated in Carbon Nanotubes by Tip-Enhanced Raman Spectroscopy". Jap. J. Appl. Phys. 45 (12): 9286–9289. Bibcode:2006JaJAP..45.9286S. doi:10.1143/JJAP.45.9286.
- ↑ Wittig G.; Pommer H.: DBP 954247, 1956
- ↑ Wittig G., Pommer H. (1959). Chem. Abstr 53: 2279. Missing or empty
|title=(help) - ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 – Labelling of ingredients". Retrieved 2014-12-22.
- ↑ US FDA: "Food Additive Status List". Retrieved 2014-12-22.
External links
- β-Carotene website by Martha Evens, School of Chemistry, University of Bristol
- Berkeley Wellness Guide to Dietary Supplements
- β-Carotene on University of Maryland Medical Center
- World's Healthiest Foods: carotenoids
- Carotene at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||
| ||||||||||||||||||||||
|