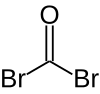
Carbonyl bromide
| |||
| Names | |||
|---|---|---|---|
| Other names
Bromophosgene, carbonic dibromide, carbonyl dibromide | |||
| Identifiers | |||
| 593-95-3 | |||
| ChemSpider | 71389 | ||
| Jmol interactive 3D | Image | ||
| PubChem | 79057 | ||
| |||
| |||
| Properties | |||
| COBr2 | |||
| Molar mass | 187.818 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 2.52 g/ml at 15°C | ||
| Boiling point | 64.5 °C (148.1 °F; 337.6 K) decomposes | ||
| reacts | |||
| Thermochemistry | |||
| 61.8 J·mol−1·K−1 (gas) | |||
| Std molar entropy (S |
309.1 J·mol−1·K−1 (gas) | ||
| Std enthalpy of formation (ΔfH |
-127.2 or -145.2 kJ·mol−1 (liquid) -96.2 or -114 kJ·mol−1 (gas) | ||
| Hazards | |||
| NFPA 704 | |||
| Related compounds | |||
| Related compounds |
Carbonyl fluoride Phosgene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Carbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound. It is a decomposition product of halon compounds used in fire extinguishers.[2]
Reactions
Carbonyl bromide is formed when carbon tetrabromide is melted and concentrated sulfuric acid is added.
In contrast to phosgene, carbonyl bromide cannot be produced efficiently from carbon monoxide and bromine. A complete conversion is not possible due to thermodynamic reasons. Additionally, the reaction
CO + Br2 <-> COBr2
processes slowly at room temperature. Increasing temperature, in order to increase the reaction rate, results in a further shift of the chemical equilibrium towards the educts (since ΔRH < 0 and ΔRS < 0).[3]
On the other hand, carbonyl bromide slowly decomposes to carbon monoxide and elemental bromine even at low temperatures.[4] It is also sensitive to hydrolysis.
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–96, 4–50, 5–26, ISBN 0-8493-0594-2
- ↑ US Occupational Safety and Health Administration (May 1996). "Common Fire Extinguishing Agents". Retrieved 2009-11-21.
- ↑ https://books.google.de/books?id=fM43Ny2eFS8C&pg=PA900&lpg=PA900&dq=carbonyl+bromide+enthalpy+of+formation&source=bl&ots=-ny9U7I-bx&sig=4XDedlqIFEK2OtXizrJaUfkBdzI&hl=de&sa=X&ei=UEgoVYCmI86uabvrgLAK&ved=0CDwQ6AEwAw#v=onepage&q=carbonyl%20bromide%20enthalpy%20of%20formation&f=false, pp. 669-671, retrieved 2015-04-11
- ↑ Katrizsky, Alan R.; Meth-Cohn, Otto; Wees, Charles W. (1995), Organic Functional Group Transformations 6, Elsevier, pp. 417–8, ISBN 978-0-08-042704-1, retrieved 2009-11-23