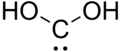Dihydroxymethylidene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dihydroxymethylidene | |||
| Systematic IUPAC name
Dihydroxymethylidene (substitutive) Dihydroxidocarbon(2•) (additive) | |||
| Other names
Carbonic(II) acid Carbonous acid | |||
| Identifiers | |||
| 71946-83-3 | |||
| ChemSpider | 11646598 | ||
| Jmol interactive 3D | Image | ||
| MeSH | Dihydroxycarbene | ||
| PubChem | 191992 | ||
| |||
| |||
| Properties | |||
| CH2O2 | |||
| Molar mass | 46.03 g·mol−1 | ||
| Related compounds | |||
| Related compounds |
Lead(II) hydroxide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Dihydroxymethylidene is a chemical compound with formula C(OH)2. There is no evidence that this compound exists in solution, but the molecule has been detected in the gas phase.[1] Many related carbenes are known, although they are often transient.[2]
Production and properties
Dihydroxymethylidene is produced in the gas phase by high vacuum flash vacuum pyrolysis of oxalic acid:
- C2O4H2 → C(OH)2 + CO2
The species is a bent molecule with an O-C-O angle of 105.6° for the C2v all trans rotamer. Although stable at 10 K, at higher temperatures it isomerizes to formic acid.
Carbonite
The conjugate base of dihydroxycarbene is the chemical formula [CO2]2−. Alkali metal salts, e.g., Li
2CO
2, K
2CO
2, and Cs
2CO
2, have been observed at 15K.[3][4]
At lower metal concentrations, salts of the monovalent anions CO−
2 were favored over CO2−
2. Carbonite was not detected when sodium was used as the metal.[4] The alkali metal carbonites obtained in the cryogenic experiments decomposed to the corresponding carbonate (with release of carbon monoxide) or oxalate.[3][4] The carbonite ion is promptly converted to carbonate in the presence of oxygen.[5][6]
The presence of carbonite ions has been proposed to be relevant to the absorption of carbon monoxide on calcium oxide and magnesium oxide[5] and on ceria.[6] In the former, it has been suggested that the carbon atom attaches coordinatively to an oxygen atom from the substrate through its free bonds.[5] In these contexts, it appears that the carbonite ion reacts with excess carbon monoxide to form an anion with the ketene structure, O=C=CO2−
2.[5]
Infrared spectroscopy data confirm earlier theoretical studies that the carbonite anion has a bent structure, with the O-C-O angle varying between 120 and 130 degrees depending on the context. The metal atoms interact with both oxygen atoms. However two geometrical arrangements for the lithium and cesium salts were detected, only one of them being symmetrical on the two oxygen atoms.[3][4]
References
- ↑ Schreiner, Peter R.; Reisenauer, Hans Peter "Spectroscopic identification of dihydroxycarbene" Angewandte Chemie, International Edition (2008, volume 47, 7071-7074. doi:10.1002/anie.200802105
- ↑ M. Jones, Jr., R. A. Moss, in "Reactive Intermediate Chemistry", Edited by R. A. Moss, M. S. Platz, M. Jones, Jr., Wiley-Interscience, Hoboken, 2004.
- 1 2 3 Zakya H. Kafafi, Robert H. Hauge, W. Edward Billups, and John L. Margrave (1983) Carbon dioxide activation by lithium metal. 1. Infrared spectra of Li+
CO−
2 , Li+
C
2O−
4 , and Li2+
2CO2−
2 in inert-gas matrices. Journal of the American Chemical Society, volume 183, pages 3886--3893. doi:10.1021/ja00350a025 - 1 2 3 4 Zakya H. Kafafi, Robert H. Hauge, W. Edward Billups, and John L. Margrave (1984), Carbon dioxide activation by alkali metals. 2. Infrared spectra of M+
CO−
2 and M2+
2CO2−
2 in argon and nitrogen matrices. Inorganic Chemistry, volume 23, pages 177-183. doi:10.1021/ic00170a013. - 1 2 3 4 M. A. Babaeva and A. A. Tsyganenko (1987), Infrared spectroscopic evidence for the formation of carbonite CO2−
2 ions in CO interaction with basic oxide surfaces Reaction Kinetics and Catalysis Letters, volume 34, issue 1, pages 9--14. doi:10.1007/BF02069193 - 1 2 Binet, Claude; Ahmed Badri; Magali Boutonnet-Kizling; Jean-Claude Lavalley (1994). "FTIR study of carbon monoxide adsorption on ceria: CO22− carbonite dianion adsorbed species". Journal of the Chemical Society, Faraday Transactions 90: 1023–1028. doi:10.1039/FT9949001023.