Collared titi
| Collared titi[1] | |
|---|---|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Primates |
| Family: | Pitheciidae |
| Genus: | Callicebus |
| Subgenus: | Torquatus |
| Species: | C. torquatus |
| Binomial name | |
| Callicebus torquatus (Hoffmannsegg, 1807) | |
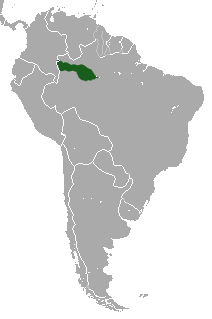 | |
| Collared Titi range | |
The collared titi, Callicebus torquatus, is a species or a closely related complex of species of titi, a type of New World monkey, from South America.
Taxonomy
At the end of the 1980s the Callicebus genus was revised from the Hershkovitz concept of three species[3] to thirteen neotropical species, with the collared titi, Callicebus torquatus, having four subspecies.[4][5] In 2001 Colin Groves elevated one of the subspecies, the Colombian black-handed titi, C. t. medemi, to Callicebus medemi and a year later Van Roosmalen et al. elevated the remaining subspecies to species.[6][7] These last changes were made with few arguments to support the changes and were apparently influenced by the increasing use of the so-called phylogenetic species concept of Cracraft, which seeks to define species as the "smallest diagnosable cluster of individual organisms within which there is a parental pattern of ancestry and descent."[8] This might work given enough information, which is usually not the case. The recent discovery of a diploid number of 16 for the black titi, Callicebus lugens, in Brazil certainly suggests that (with the previously known 2n=20 of another, unidentified population of C. torquatus) there are at least two species in this complex.[9] But whether the Lucifer titi, Callicebus lucifer, or the Colombian black-handed titi, Callicebus medemi, are good species from this complex is in doubt.[10] They are probably subspecies of Callicebus torquatus. Nevertheless, in this treatment C. torquatus is used in the sense of Hershkovitz (1990)[4] until the systematics of this species complex becomes clearer.
Physical description
Five adults weighed an average of 1462 g (range 1410–1722 g) with a head-body length of around 290–390 mm and a tail length of about 350–400 mm.[4] The face has very little hair, being limited to sparse short white hairs over a black skin. There is no sexual dimorphism, although the male has canines a bit longer than the female. The species has the smallest karyotype known for primates, 2n=16 recently described by Bonvicino et al.[11]
The species' pelage is uniformly reddish brown or blackish brown, the tail is blackish mixed with some reddish hairs; hands and feed whitish or dark brown. This pelage contrasts in all of the subspecies with a band of white hair which extends upward from the chest and follows the neck, prolonging itself to the ears. This extension to the ears is weak in Callicebus torquatus torquatus, a subspecies not confirmed for Colombia and different from the other subspecies which have white extending to the base of the ears. The other subspecies are differentiable from the above subspecies as follows:
- Callicebus torquatus lugens, the pelage is generally blackish mixed with dark brownish and some reddish brown hairs on the back and the flanks. Hands are white or yellowish.
- Callicebus torquatus lucifer, the pelage is basically blackish but intermixed with many hairs on the back (extending to the top of the crown) and flanks with many reddish brown hairs, giving the animal a definite reddish appearance in the sunlight.
- Callicebus torquatus medemi, the pelage is predominantly black variable looking like lugens or like lucifer but with the white chest and the hands are blackish instead of whitish.
Geographic distribution and habitat
The collared titi is found throughout lowland Colombian Amazonia up to about 500 m of altitude in Putumayo and probably about the same in Caquetá. The species has been observed on the left bank of the Guayabero River,[12], where it was collected in 1959 by Jorge Hernández Camacho, both in La Macarena National Park, and recently it was observed by the Colombian biologist Rocío Palanco north of the Guayabero above La Cordillera de los Picachos National Park. The species is found in the Vichada selva between the Vichada and Guaviare Rivers and the northernmost Colombian population extends north of the Vichada River, reaching the middle Tomo River, where it probably extends to the upper Tomo, although this needs to be confirmed.
The collared titi is not found on the lower Tomo or lower Tuparro River nor on the north bank of the lower Vichada River, contrary to the distribution map of Hershkovitz.[5][4] This error is due to the collection of a specimen by the English ornithologist Cherrie in about 1904 from Maipures, which may have been a captive animal obtained in the village, since extensive and concerted efforts have failed to identify it for the entire area mentioned above; nor is it known by locals for this area. The nearest titi monkeys from Maipures in Colombia are found on the middle Tuparro River and south of the lower Vichada River.[13]
Outside of Colombia this species extends from the Napo River northward throughout the Ecuadorian[14] and Peruvian Amazonia and throughout southern Venezuela to the Río Branco and the lower Río Negro in Brazil. South of the Amazon it is found west of the Río Purús to the Yavarí River.[4]
The collared titi is seen most frequently in well-developed, tall forest with a closed canopy, usually over terra firme, but not exclusively so. The species also enters extensive várzea forest, especially if the forest is tall and well-developed.[15] Such várzea forest contrasts with the habitat needs of the coppery titi, which also uses várzea forest and more commonly so. But the coppery titi survives in low, vine-covered, "poor" forest where the collared titi is rarely found. It is also known from some gallery forest, north of the Vichada River, although this seems to be the exception for the species, which is much more extensively known from closed-canopy forest to the south. Contrary to Kinzey & Gentry,[16] the forest habitat of the collared titi grows on a variety of substrates, varying from white sand through many types of clay soils.[15]
Natural history
This species was studied in Peru by Kinzey (1975, 1976, 1977a, 1977b, 1977c, 1978, 1981, Kinzey et al., 1977, Kinzey & Gentry, 1979, Kinzey & Wright, 1982, Kinzey & Robinson, 1981, 1983) and by Easley (1982), Easley & Kinzey (1986), Starin (1992), Milton & Nessimain (1984), Robinson et al. (1987) (a review article). In Colombia the species has been studied by Defler (1983b, 1994a, 2003), Forero (1985), Palacios & Rodríguez (1994) and Palacios et al. (1997).
Social groups are made up of a monogamous pair and one or two of its young. A count of ten groups in Vichada yielded an average of 3.5 per group.[17] Occasionally groups of five are seen and unpaired individuals ("floaters") can also be detected from time to time. Second year youngsters usually leave the group, although they may make it into the third year before leaving. These young animals sometimes appear, moving peripherally to the group and then disappear again to move alone.
Measured home ranges have varied from about 15–25 ha. Appropriate habitat contains 4–5 groups/km2 (14 + "floaters"), which may add another 8–10 individuals to the total ecological density/km2. The average day range calculated by Kinzey (1977) and Kinzey et al. (1977) was 819.4 m (n=22 days) for a research project in Peru and at the Estación Biológica Caparú the average was 807.2 m (range 513.7 – 1070 m, n=26).[18][19]
Easley calculated a time budget based on 400 hours of observation as 62.7% rest, 16.5% moving, 16.1% feeding, 2.7% grooming, 1.6% playing and 0.3% vocalizing.[20] Palacios & Rodríguez calculated 54.3% rest, 22.9% moving, 17.6% feeding, 4.07% grooming, 0.41% playing, and 0.42% vocalizing based on 240 hours of observation.[18]
Easley analyzed the locomotive and positional behavior of the species showing that it is a generalized quadruped using quadrupedal walking and running about 66.8% of the time.[20] This species also engages in active jumping (23.9% of the time) and climbing 9.1% of the time). Sitting (62%3% is the most common posture, followed by lying (16.1%), walking (10.4%), jumping (4%), vertical clinging (3.1%), climbing (1.5%), running (0.8%), hanging suspended by the back legs (0.8%), horizontal clinging (0.7%) and standing 0.2%). If postures of locomotor behaviors are excluded from this analysis then the scores were sitting (74.8%), lying (19.3%), vertical clinging (3.7%), hanging suspended from the hind foot (0.9%), horizontal clinging (0.8%) and standing (0.2%).[20] Previously Kinzey & Rosenberger had pointed out that these animals fit into the "clinging and leaping" group of primates.[21] Groups of collared titis sleep on top of large branches of emergent trees, frequently a bit above the level of the main canopy.[22]
Diet
Although fruits are the major portion of this primate's diet, invertebrates and leaves are also consumed to a smaller degree. Lepidopteran larva, spiders and orthopterans are especially eaten with relish and probably occasional small lizards, judging by the hunting preferences of a tame, free-ranging adult female, which lived at the Caparú Biological Research Station on the lower Apaporis River.[13]
Kinzey found the following range of dietary preference during his 135 hours study in Peru: 14% Clarisia racemosa (Moraceae); 13% unidentified (Guttifereae); 7% Pithecellobium sp. (Convolvulaceae); Jessenia bataua (Arecaceae); Psychotrian axillaris (Rubiaceae); Guatteria elata (Annonaceae); Virola sp. (Myristicaceae).[23]
Easley identified frequency of item choice in the diet of the same groups as above: 74.1% fruits, 15.8% insects, 8.8% leaves, 0.6% buds and flowers and 0.1% other. Of the 57 fruit species identified, the palm tree, Jessenia polycarpa, was the most commonly eaten in 22.7% of the feeding observations.[20] The following lists the range of preference observed in this study: 22.7% Jessenis polycarpa (Arecaceae), 7.9% Ocotea no. 1 (Lauraceae); 6.6% Tachigalia sp. (Caesalpiniaceae); 5.9% Beilschmiedia sp. (Lauraceae); 5.8% Ocotea no. 2 (Lauraceae); 4.8% unidentified; 3.5% unidentified; 3.5% Guatteria sp. (Annonaceae); 3.4% Annona sp. (Annonaceae); 2.4% unidentified; 2.0% unidentified; Guatteria sp. (Annonaceae); 1.9% Duguetia sp. (Annonaceae).[20]
Palacios & Rodríguez and Palacios et al. identified 62 species from 32 plant familias in the diet of a study group of black titis in the Estación Biológica Caparú in eastern Colombia. The preference values of each family, according to species utilized is as follow: Myristicaceae (25.02%); Euphorbiaceae (15.28%); Moraceae (14.37%); Arecaceae (8.68%); Caesalpiniaceae (7.85%) Rubiaceae (5.10%); Chrysobalanaceae (4.41%); Annonaceae (4.19%); Cecropiaceae (4.03%); Araceae (1.95%); Elaeocarpaceae (1.78%); Dilleniaceae (1.69%), Combretaceae (1.17%), Apocynaceae (1%); Aquifoliaceae (1%), Meliaceae (0.88%); Sapotaceae (0.85%); Burseraceae (0.81%); Apocynaceae (0.67%); Monimiaceae (0.23%); Piperaceae (0.22%); Melastomaceae (0.18%); Humiriaceae (0.13%) Celastracezae (0.11%); Myrtaceae (0.09%); Lecythidaceae (0.08%); Aquifoliaceae (0.07%); Sterculiaceae (0.07%); Solanaceae (0.05%); Clusiaceae (0.02%).[18][19]
The most important species consumed during six months in this study are listed as follows: 13.88% Sandwithia heterocalyx (Euphorbiaceae); 10% Virola melinonii (Myristicaceae); 8.35% Iryanthera ulei (Myristicaceae); 7.06 Oenocarpus bataua (Arecaceae); 6.53% Heterostemon conjugatus (Caesalbiniaceae); 5.10% Coussarea sp. (Rubiaceae); 5.02% Ficus sp. (Moraceae); 4.53% Iryanthera crassifolia (Myristicaceae); 3.84% Helicostylis tomentosa (Moraceae); 3.39% Brosimum rubescens (Moraceae).[19]
Reproduction
The estrus cycle seems to be about 16 days, based on observations of 14 cycles of a tame, free-ranging female which lived at the Estación Biológica Caparú (Vaupés, Colombia). During the period of receptivity (which lasts 2–3 days) the black labia and the clitoris became swollen and hard and behavior changes occurred. During the receptive period the female became much more affectionate towards its human "parents", purred loudly, somewhat like a cat and crouched in a lordic position when the base of the tail was stimulated. Contrarywise to her increased affection towards her perceived "family unit" (or two humans), she became much more aggressive than normal towards any "outsiders" (i.e. other human beings). During estrus the female tongue-flicked frequently, using this signal in two opposite contexts; she tongue-flicked as she attempted to approach her favorite humans while she also tongue-flicked as a preliminary to attach on other (especially male) humans.[13]
One recognizable pair at the Estación Biológica Caparú had been observed together for 14 years and was said to be still together at least four years more after this author had left. During the 14 years the pair produced 10 young, all of which survived the first year. During four years no young were produced.
In Vichada young are usually produced in December or early January.[24] This is a difficult season with sharply reduced fruit resources for many animals in this part of the country (which has an annual precipitation of about 2400 mm; a long dry season is just taking hold and January and February present only a very few millimeters of precipitation for each month. A close analysis of the diet of the black titi here would be interesting, inasmuch as it would serve to identify the resources which allow the species to have this birth pattern.
On the Guayabero River near La Macarena the birth season is apparently about the same time as in Vichada.[25] On the lower Apaporis River in Vaupés with about 3815 mm of precipitation throughout the year, the birth season is also centered around December, although some outlying births are known as early as the first of October. Nevertheless, the birth season is the same as the other two sites, despite the lack of a strong dry season. However, we know that fleshy fruits are beginning to increase from their yearly low during this time, so the question of resource use by the species remains very interesting.[13]
Robinson et al. also report a birth season of December to January for the species in Peru at 4ºS.[26] Why this specific birth season should be chosen by the species in such widely divergent places both north and south of the equator with different phenological cycles must remain for the moment an open question. The newborn quickly acclimates to being carried by the male, and usually goes to the female for nursing only.[26]
Communication
The collared titi is very affectionate within the family unit, but the adult pair is aggressive towards neighboring pairs. The most common interaction with neighbors is counter-singing of the pairs, where one pair waits listening while the other pair vocalizes their duet, later the listeners answer, while the first vocalizers listen. There are instances when two pairs interchange vocalizations from very close together or from almost the same place in the forest. Sometimes these emotional interactions may finish in chases by the pair or an individual against the others. Rodríguez & Palacios (1994) found evidence of different types of agonistic interactions between different pairs.[27] For example, a habituated group at Estación Biológica Caparú (perhaps for historic reasons) treats one pair of neighbors differently from another pair. The group studied at Estación Biológica Caparú interacts via counter-singing with the young pair which took territory left by the study pair, while the group both counter-sings and is aggressive in other ways towards a different neighboring group. Perhaps the young pair is made up of one of the offspring of the other group?
Between group members a lot of affection is evident including much contact. The affection is probably one of the mechanisms which maintains the ties in these associations which apparently last until one of the members of the pair dies.
Vocalizations of this species are very complex, especially a long-call display utilized by these animals, perhaps to regulate spacing and defined territory.[26] Surprisingly, experimental playback of solo male calls caused the owners of a particular territory to move away from the recording, and recordings of duetting caused the territory owners to duet in return and to travel parallel to the speaker.[28] However, any approximate sound stimulus can cause duetting of territory owners, and many direct observations of duetting neighbors were observed to cause the territorial owners to move towards the calling, where they sometimes confronted each other across a small space.[13] Lone individuals in the established territory of another pair do not normally vocalize, since they may be vigorously attacked if the "owners" of the territory find them.
There is some evidence that titis not only can determine sex from a long call but can identify duetting individuals, so it should perhaps not be surprising that a resident pair could distinguish a recording from a live monkey and move away from it.
A human-raised and newly matured female black titi on first shouting, attracted the resident forest group to come closer until they became accustomed to her presence, although they always answered her calling with their duetting, later neither coming closer nor moving away. The female's vocalizations sometimes attracted several individual males in short order, which attempted to duet with the female. Since the female had been raised by humans, she did not show interest in duetting with the newly appeared males nor in establishing a relationship with them, and the males eventually desisted and left. The only exception to this was one male which attempted to establish a relationship during two years before giving up and leaving during an accidental 26-day absence of the female when she became inadvertently lost in the forest.[13]
Some vocalizations of black titi are listed here: (1). Morning duet – the most commonly heard vocalization of the pair, singing in duet, complex and utilized to defend territory; it is interchanged with neighboring groups as counter-singing; (2) danger peep – various soft, high-pitched peeps but sometimes low intensity, advising of danger; very difficult to localize; (3) purr – sounds very much like a cat's purr; used by all members of the group to show contentment, affection or request for food, grooming or contact; (4) rough growl – given by young animals when complaining of rain or when greeting adults; (5) sharp scream – when fighting to express extra disgust; (6) play growl – low, gargling growl used in play and changing in tone, terminating in interrogative tone; (7) soft whine – especially young animals but also adults when requesting something of another such as food or while grooming another; (8) bark – loud, sharp and sudden bark when molested by the unwelcome close presence of other larger primates such as Lagothrix, Cebus, Ateles or raptors.[13]
Individuals of both sexes occasionally mark their chests with pungent wadded leaves, rubbing the leaf up onto the throat and chin to the mouth, where the wad is wetted and rubbed down again, repeated various times while looking up into the air. One wild male did this as he approached the tame estrus female, who was near a building, after this male had left the forest and while walking on the elevated poles which had been set up for monkey travel. Another foraging female marked herself in the presence of an observing human who was 20 m from her.
Displays are similar to the coppery titi, which were first described by Moynihan (1966, 1967, 1976a). Some displays are listed here: (1) piloerection – agonistic; excited state when attacked or attacking; during danger; (2) arched-back – agonistic; before some attacks or when threatened; position held for several seconds; (3) tail twinning – when duetting or resting the pair often wind their tails around each other's tail; (4) tongue flicking – in two contexts; aggressive just before attack or as space reducer towards mate and probably just before copulation (hand-raised female at EBC tongue flicks at human "parent", especially at height of estrus cycle; (5) chest rubbing – using a wadded leaf the individual rubs from throat to chest after first wetting the leaf with saliva; performed in presence of human observer; nervousness.[13]
Kinzey et al. observed play behavior only between the infant and male and between two juveniles.[29] In Caparú the adult female occasionally exhibited play behavior towards her human "parents" with slight head shakes and sideways jumps on the ground. Agonistic behavior is common between neighboring groups and can sometimes results in fights, although usually the aggression is limited to intergroup vocalization.
Interspecific interactions and predators
The collared titi usually attempts to move out of the path of passing troops of brown woolly monkey or tufted capuchin, although sometimes the small monkeys give a burst of loud and aggressive-sounding vocalization ("bark") when they are approached closely by the larger species. Titis frequently hides and shows much caution towards raptors. Being frightened causes them to give alarm peeps, probably because they must be especially alert to predators. A margay was detected alongside a dead collared titi during recent censuses on the Purité River in Colombia, although the monkey was not freshly killed. The local group was no longer observed after this.
Conservation status
The collared titi is not considered to be endangered, but where there are many colonists this primate tends to disappear, due to deforestation. The species is commonly hunted and eaten by indigenous peoples or used as bait for hunting larger carnivores or for fishing; however, where there is plenty of forest meat the species is found commonly close to Indian settlements. The species is classified Least Concern (formerly LR) in the IUCN Red List.[2] Actually the Colombian black-handed titi may be slightly endangered due to its presence in a zone of heavy colonization, but presently it is classified LC with the other subspecies. Fortunately the Colombian black-handed titi is found in La Paya National Park in Colombia, although the park itself is very vulnerable to surrounding colonization. The Lucifer titi is protected in Colombia by Amacayacu National Park and Cahuinarí National Park, while the black titi may be protected in Chiribiquete National Park and El Tuparro National Park and in the two biological preserves Nukak and Puinawai. Actually a clarification of the taxonomic status of many populations is needed to be able to monitor their conservation state.
References
- ↑ Groves, C.P. (2005). Wilson, D.E.; Reeder, D.M., eds. Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Baltimore: Johns Hopkins University Press. p. 146. OCLC 62265494. ISBN 0-801-88221-4.
- 1 2 Veiga, L.M. & Boubli, J.-P. (2008). Callicebus torquatus. In: IUCN 2008. IUCN Red List of Threatened Species. Retrieved 3 January 2009.
- ↑ Hershkovitz, P (1963): A systematic and zoogeographic account of South American titi monkeys, genus Callicebus (Cebidae) of the Amazonas and Orinoco river basins, Mammalia, 27(1):1-80.
- 1 2 3 4 5 Hershkovitz, P (1990): Titis, new world monkeys of the genus Callicebus (Cebidae, Platyrrhini): a preliminary taxonomic review,Fieldiana (Zoology, New Series, no. 55):1-109-
- 1 2 Hershkovitz, P. (1988): Origin, speciation, dispersal of South American titi monkeys, genus Callicebus (family Cebidae, Platyrrhini), Proceedings of the Academy of Natural Sciences of Philadelphia, 140(1):240-272.
- ↑ Groves 2001
- ↑ Van Roosmalen, M.G.M.; Van Roosmalen, T.; Mittermeier, R.A. (2002). "A taxonomic review of the titi monkeys, genus Callicebus Thomas, 1903, with the description of two new species, Callicebus bernhardi and Callicebus stephennashi from the Brazilian Amazon" (PDF). Neotropical Primates 10 (Suppl.): 1–52. Retrieved 2011-01-23.
- ↑ Cracraft 1983, pp. 159–187
- ↑ Barros, R. M. S.; Pieczarka, J. C.; Brigido, M. D. C. O.; Muniz, J. A. P. C.; Rodrigues, L. R. R.; Nagamachi, C. Y. (2004). "A new karyotype in Callicebus torquatus (Cebidae, Primates)". Hereditas 133 (1): 55–8. doi:10.1111/j.1601-5223.2000.t01-1-00055.x. PMID 11206854.
- ↑ Defler 2010
- ↑ Bonvicino, C. R.; Penna-Firme, V. E. R.; Do Nascimento, F. I. C. F.; Lemos, B.; Stanyon, R.; Seuánez, H. E. C. N. (2003). "The Lowest Diploid Number (2n = 16) yet Found in Any Primate: Callicebus lugens (Humboldt, 1811)". Folia Primatologica 74: 141. doi:10.1159/000070647.
- ↑ Olivares, 1962
- 1 2 3 4 5 6 7 8 Defler, T. R. 2003. Primates de Colombia. Conservation International, Bogota.
- ↑ Ulloa, 1986
- 1 2 Defler, 1994a
- ↑ Kinzey, 1978
- ↑ Defler, T. R. (1983). "A remote park in Colombia". Oryx 17: 15. doi:10.1017/S0030605300018330. ISSN 1365-3008.
- 1 2 3 Palacios, 1994
- 1 2 3 Palacios et al., 1997
- 1 2 3 4 5 Easley, 1982
- ↑ Kinzey, 1975
- ↑ Kinzey, 1981
- ↑ Kinzey, 1977
- ↑ Defler, T.R. "Some population characteristics of Callicebus torquatus legens (Humboldt, 1812) (Primates, Cebidae) in eastern Colombia". Lozania 38: 1–9.
- ↑ Hernández-Camacho 1976, pp. 35–69
- 1 2 3 Robinson et al., 1987
- ↑ Rodríguez & Palacios, 1994
- ↑ Kinzey & Robinson, 1983
- ↑ Kinzey et al., 1977
- Books cited
- Battelle Seattle Research Center; Institute of Laboratory Animal Resources (U.S.); Institute of Laboratory Animal Resources (U.S.); Committee on Conservation of Nonhuman Primates (1976). Thorington, R.W.; Heltne, P.G., eds. Neotropical primates: field studies and conservation : proceedings of a symposium on the distribution and abundance of neotropical primates. Washington, D.C.: National Academy of Sciences. ISBN 0-309-02442-0.
- Hernández-Camacho, J.I.; Cooper, G.W. (1976). "The non-human primates of Columbia". pp. 35–69. Missing or empty
|title=(help)
- Hernández-Camacho, J.I.; Cooper, G.W. (1976). "The non-human primates of Columbia". pp. 35–69. Missing or empty
- Johnston, R.F.; Power, D.M. (ed.). Current Ornithology. Vol. 1. New York: Plenum Press. ISBN 0-306-41780-4. OCLC 181794456.
- Cracraft, J. (1983). "Species concepts and speciation analysis". pp. 159–187. Missing or empty
|title=(help)
- Cracraft, J. (1983). "Species concepts and speciation analysis". pp. 159–187. Missing or empty
- Defler, T.R. (2010). Historia Natural de los Primates Colombianos [Natural History of the Colombian Primates]. Bogotá, Colombia: Universidad Nacional de Colombia.
- Groves, C.P. (2001). Primate Taxonomy. Smithsonian Institution Press. ISBN 1-56098-872-X.
External links
| Wikispecies has information related to: Collared Titi |
- Callicebus torquatus, Collared Titi Monkey on Digimorph
