Calcium hydroxide
 | |
| Names | |
|---|---|
| IUPAC name
Calcium hydroxide | |
| Other names
Slaked lime Milk of lime Caustic Soda Calcium(II) hydroxide Pickling lime Hydrated lime Portlandite Calcium hydrate | |
| Identifiers | |
| 1305-62-0 | |
| ChEBI | CHEBI:31341 |
| ChemSpider | 14094 |
| EC Number | 215-137-3 |
| Jmol interactive 3D | Image Image |
| KEGG | D01083 |
| PubChem | 14777 |
| RTECS number | EW2800000 |
| UNII | PF5DZW74VN |
| |
| |
| Properties | |
| Ca(OH)2 | |
| Molar mass | 74.093 g/mol |
| Appearance | white powder |
| Odor | odorless |
| Density | 2.211 g/cm3, solid |
| Melting point | 580 °C (1,076 °F; 853 K) (loses water, decomposes) |
| 0.189 g/100 mL (0 °C) 0.173 g/100 mL (20 °C) 0.066 g/100 mL (100 °C) | |
| Solubility product (Ksp) |
5.5×10−6 |
| Solubility | Soluble in glycerol and acids. Insoluble in alcohol. |
| Acidity (pKa) | 12.4 |
| Basicity (pKb) | 2.37 |
| Refractive index (nD) |
1.574 |
| Thermochemistry | |
| Std molar entropy (S |
83 J·mol−1·K−1[1] |
| Std enthalpy of formation (ΔfH |
−987 kJ·mol−1[1] |
| Hazards | |
| Safety data sheet | See: data page [2] |
| R-phrases | R22, R34 |
| S-phrases | (S2), S24 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose) |
7340 mg/kg (oral, rat) 7300 mg/kg (mouse) |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 15 mg/m3 (total) 5 mg/m3 (resp)[3] |
| REL (Recommended) |
TWA 5 mg/m3[3] |
| IDLH (Immediate danger |
N.D.[3] |
| Related compounds | |
| Other cations |
Magnesium hydroxide Strontium hydroxide Barium hydroxide |
| Related bases |
Calcium oxide |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| | |
| Infobox references | |
Calcium hydroxide, traditionally called slaked lime, is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is obtained when calcium oxide (called lime or quicklime) is mixed, or "slaked" with water. It has many names including hydrated lime, caustic lime, builders' lime, slack lime, cal, or pickling lime. Calcium hydroxide is used in many applications, including food preparation. Limewater is the common name for a saturated solution of calcium hydroxide.
Properties
Calcium hydroxide is relatively soluble in water, with a solubility product Ksp of 5.5 × 10−6. It is large enough that it will partially dissolve and release hydroxyl anions (OH-) in solution according to the following reaction:
- Ca(OH)2 → Ca2+ + 2 OH-
At ambient temperature, about 1 g of calcium hydroxide (portlandite) can dissolve in pure water to produce an alkaline solution with a pH of about 12.5. Calcium hydroxide solutions can therefore cause severe chemical burns. The solubility of calcium hydroxide also strongly depends on pH value. At high pH value, in the presence of alkali hydroxides (NaOH, KOH), such as in fresh cement water, calcium hydroxide solubility drastically drops.
A suspension of fine calcium hydroxide particles in water is called milk of lime. The solution is called limewater and is a medium strength base that reacts with acids and can attack some metals such as aluminium (amphoteric hydroxide dissolving at high pH) while protecting other metals from corrosion such as iron and steel by passivation of their surface. Limewater turns milky in the presence of carbon dioxide due to formation of calcium carbonate, a process called carbonatation:
- Ca(OH)2 + CO2 → CaCO3 + H2O
When heated to 512 °C, the partial pressure of water in equilibrium with calcium hydroxide reaches 101 kPa (normal atmospheric pressure), which decomposes calcium hydroxide into calcium oxide and water.[4]
- Ca(OH)2 → CaO + H2O
Structure, preparation, occurrence

Calcium hydroxide adopts a polymeric structure, as do the related hydroxides of the alkaline earth metals. The packing resembles the cadmium iodide motif with layers of octahedral Ca centres. Strong hydrogen bonds exist between the layers.[5]
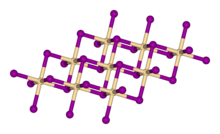
Calcium hydroxide is produced commercially by treating lime with water:
- CaO + H2O → Ca(OH)2
In the laboratory it can be prepared by mixing aqueous solutions of calcium chloride and sodium hydroxide. The mineral form, portlandite, is relatively rare but can be found in some volcanic, plutonic, and metamorphic rocks. It has also been known to arise in burning coal dumps. CaOH has been detected in the atmosphere of S-type stars.[6]
Uses
One significant application of calcium hydroxide is as a flocculant, in water and sewage treatment. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. This application is enabled by the low cost and low toxicity of calcium hydroxide. It is also used in fresh water treatment for raising the pH of the water so that pipes will not corrode where the base water is acidic, because it is self-regulating and does not raise the pH too much.
It is also used in the preparation of ammonia gas, using the following reaction:
Ca(OH)2 + 2NH4Cl → 2NH3 + CaCl2 + 2H2O
Another large application is in the paper industry, where it is an intermediate in the reaction in the production of sodium hydroxide. This conversion is part of the cuasticizing step in the Kraft process for making pulp.[5] In the cuasticizing operation burned lime is added to green liquor which is a solution primarily of sodium carbonate and sodium sulfate produced by dissolving smelt, which is the molten form of these chemicals from the recovery furnace.
Niche uses
Calcium hydroxide is produced on a large scale, is easily handled and is generally inexpensive. Numerous niche applications are in use. A partial listing follows:
- In life support systems as a carbon dioxide scrubber, particularly in closed-circuit diving re-breathers such as the US Navy LAR V or MK-16, where the more caustic lithium hydroxide is deemed too risky due to inhaled dust, combat handling, or generation of caustic "slurry" in flooding events
- An ingredient in whitewash, mortar, and plaster
- In road construction, to improve the quality of excessively plastic subgrade soils
- To fill the root canal for the first stage of endodontic therapy (it is then replaced by rubber)
- As an additive to sea water to reduce atmospheric CO2 and mitigate the greenhouse effect.[7] However, producing the required calcium hydroxide generates CO2 and requires large amounts of energy which is typically supplied by burning coal or natural gas, generating additional CO2.
- In the production of metals, lime is injected into the waste gas stream to neutralize acids, such as fluorides and chlorides prior to being released to atmosphere.
- An alkali used as a lye substitute in no-lye hair relaxers
- A chemical depilatory agent found in most hair removal creams (for example Nair)
- In Bordeaux mixture to precipitate copper hydroxide from the solution and to maintain a high pH in order to form a long-lasting fungicide
- In lime-sulfur, it is mixed with sulfur and boiled in water for an hour. The ratio by mass of Ca(OH)2:Sulfur:water is about (1):(1.7):(8.6). Diluted (1:25) lime-sulfur is used as a dip to combat sarcoptic mange. Against fungus on plants it is used in various concentrations.
- In the petroleum refining industry for the manufacture of additives to oils (salicatic, sulphatic, fenatic)
- In the chemical industry for manufacture of calcium stearate
- In the petrochemical industry for manufacturing solid oil of various marks
- In the manufacture of brake pads
- In manufacturing the trademarked compound "Polikar", an antifungal and antimicrobial preservative for vegetables in storage
- For preparation of dry mixes for painting and decorating
- In manufacturing mixes for pesticides
- In the manufacture of ebonite
- As a calcium supplement and pH/carbonate buffer (known as Kalkwasser lit. trans. lime-water) for the aquaculture of corals in reef aquaria.
- As a natural "alternative" insecticide, most crawling insects are killed by its touch, including ticks, fleas, beetles and grubs.
Ancient Celtic use
According to Diodorus Siculus:
"The Gauls are tall of body with rippling muscles and white of skin and their hair is blond, and not only naturally so for they also make it their practice by artificial means to increase the distinguishing colour which nature has given it. For they are always washing their hair in limewater and they pull it back from the forehead to the nape of the neck, with the result that their appearance is like that of Satyrs and Pans since the treatment of their hair makes it so heavy and coarse that it differs in no respect from the mane of horses."
Food industry
Because of its low toxicity and the mildness of its basic properties, slaked lime is widely used in the food industry to:
- clarify raw juice from sugarcane or sugar beets in the sugar industry, (see carbonatation)
- process water for alcoholic beverages and soft drinks
- pickle cucumbers and other foods
- make Chinese century eggs
- make corn tortillas (it helps the corn flour (masa) bind together) (see nixtamalization)
- clear a brine of carbonates of calcium and magnesium in the manufacture of salt for food and pharmaceutical uses
- fortify (Ca supplement) fruit drinks, such as orange juice, and infant formula
- aid digestion (called Choona, used in India in paan, a mixture of areca nuts, calcium hydroxide and a variety of seeds wrapped in betel leaves)
- substitute for baking soda in making papadam.
Native American uses

In Spanish, calcium hydroxide is called cal. Corn cooked with cal (nixtamalization) becomes hominy (nixtamal), which significantly increases the bioavailability of niacin, and it is also considered tastier and easier to digest.
In chewing coca leaves, calcium hydroxide is usually chewed alongside to keep the alkaloid stimulants chemically available for absorption by the body. Similarly, Native Americans traditionally chewed tobacco leaves with calcium hydroxide derived from burnt mollusk shells to enhance the effects. It has also been used by some indigenous American tribes as an ingredient in yopo, a psychedelic snuff prepared from the beans of some Anadenanthera species.[8]
Asian uses
Calcium hydroxide is typically added to a bundle of areca nut and betel leaf to keep the alkaloid stimulants chemically available to enter the bloodstream via sublingual absorption.
Afghan uses
It is used in making naswar (also known as nass or niswar), a type of dipping tobacco made from fresh tobacco leaves, calcium hydroxide (chuna), and wood ash. It is consumed most in the Pathan diaspora, Afghanistan, Pakistan, India, Bangladesh and also in Sweden and Norway. Villagers also use calcium hydroxide to paint their mud houses in Afghanistan, Pakistan and India.
Health risks
Unprotected exposure to Ca(OH)2 can pose health risks, so it should be limited. It can cause severe skin irritation, chemical burns, blindness, or lung damage. See MSDS.[2]
See also
- Baralyme (carbon dioxide absorbent)
- Lime mortar
- Lime plaster
- Plaster
- Magnesium hydroxide (less alkaline due to a lower solubility product)
- Soda lime (carbon dioxide absorbent)
References
- 1 2 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A21. ISBN 0-618-94690-X.
- 1 2 "MSDS Calcium hydroxide" (PDF). Retrieved 2011-06-21.
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0092". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Halstead, P.E.; Moore, A.E. (1957). "The Thermal Dissociation Of Calcium Hydroxide". Journal of the Chemical Society 769: 3873. doi:10.1039/JR9570003873.
- 1 2 Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ Jørgensen, Uffe G. (1997), "Cool Star Models", in van Dishoeck, Ewine F., Molecules in Astrophysics: Probes and Processes, International Astronomical Union Symposia. Molecules in Astrophysics: Probes and Processes 178, Springer Science & Business Media, p. 446, ISBN 079234538X.
- ↑ O'Driscoll, Catherine (21 July 2008). "A dash of lime – a new twist that may cut CO2 levels back to pre-industrial levels". Chemistry. PhysOrg.com. Retrieved 20 November 2010.
- ↑ de Smet, Peter A. G. M. (1985). "A multidisciplinary overview of intoxicating snuff rituals in the Western Hemisphere". Journal of Ethnopharmacology 3 (1): 3–49. doi:10.1016/0378-8741(85)90060-1.
External links
- National Organic Standards Board Technical Advisory Panel (2002-04-04). "NOSB TAP Review: Calcium Hydroxid" (PDF). Organic Materials Review Institute. Archived from the original (.PDF) on 2007-10-31. Retrieved 2008-02-05.
- CDC – NIOSH Pocket Guide to Chemical Hazards – Calcium Hydroxide
- MSDS Data Sheet
