Butanol
Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C4H9OH. There are four possible isomeric structures for butanol, from a straight-chain primary alcohol to a branched-chain tertiary alcohol;[1] all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, n-BuOH, and i-BuOH). These are n-butanol, 2-butanol, tert-butanol, and isobutanol. It is primarily used as a solvent, as an intermediate in chemical synthesis, and as a fuel. It is sometimes also called biobutanol when produced biologically.
Isomers
The unmodified term butanol usually refers to the straight chain isomer with the alcohol functional group at the terminal carbon, which is also known as n-butanol or 1-butanol. The straight chain isomer with the alcohol at an internal carbon is sec-butanol or 2-butanol. The branched isomer with the alcohol at a terminal carbon is isobutanol or 2-methyl-1-propanol, and the branched isomer with the alcohol at the internal carbon is tert-butanol or 2-methyl-2-propanol.
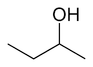 |
 |
 | |
| n-butanol | sec-butanol | isobutanol | tert-butanol |
Butanol isomers, due to their different structures, have somewhat different melting and boiling points. n-Butanol and isobutanol have limited solubility, sec-butanol has substantially greater solubility, while tert-butanol is fully miscible with water. This is because all alcohols have a hydroxyl group which makes them polar which in turn tends to promote solubility in water. At the same time, the carbon chain of the alcohol resists solubility in water. Methanol, ethanol, and propanol are fully miscible with water, while n-butanol is only moderately soluble because of the balance between the two opposing solubility trends.
Toxicity
Like many alcohols, butanol is considered toxic. It has shown low order of toxicity in single dose experiments to laboratory animals.[2][3] and is considered safe enough for use in cosmetics. Brief, repeated overexposure with the skin can result in depression of the central nervous system, as with other short-chain alcohols. Exposure may also cause severe eye irritation and moderate skin irritation. The main dangers are from prolonged exposure to fumes. In extreme cases this includes suppression of the central nervous system and even death. Under most circumstances, butanol is quickly metabolized to carbon dioxide. It has not been shown to damage DNA or cause cancer.
Uses
Biobutanol
Butanol is considered as a potential biofuel (butanol fuel). Butanol at 85 percent strength can be used in cars designed for gasoline (petrol) without any change to the engine (unlike 85% ethanol), and it contains more energy for a given volume than ethanol and almost as much as gasoline, so a vehicle using butanol would return fuel consumption more comparable to gasoline than ethanol. Butanol can also be used as a blended additive to diesel fuel to reduce soot emissions.[4]
Other uses
Butanol sees use as a solvent for a wide variety of chemical and textile processes, in organic synthesis and as a chemical intermediate. It is also used as a paint thinner and a solvent in other coating applications where it is used as a relatively slow evaporating latent solvent in lacquers and ambient-cured enamels. It finds other uses such as a component of hydraulic and brake fluids.[5]
Butanol is used in the synthesis of 2-Butoxyethanol[6]
It is also used as a base for perfumes, but on its own has a highly alcoholic aroma.
Salts of butanol are chemical intermediates; for example, alkali metal salts of tert-butanol are tert-butoxides.
Production
Since the 1950s, most butanol in the United States is produced commercially from fossil fuels. The most common process starts with propene (propylene), which is run through a hydroformylation reaction to form butyraldehyde, which is then reduced with hydrogen to 1-butanol and/or 2-butanol. Tert-butanol is derived from isobutane as a co-product of propylene oxide production. Butanol can also be produced by fermentation of biomass by bacteria. Prior to the 1950s, Clostridium acetobutylicum was used in industrial fermentation processes producing butanol. Research in the past few decades showed results of other microorganisms that can produce butanol through fermentation.
See also
References
- Merck Index, 12th Edition, 1575.
- ↑ Atsumi, S.; Hanai, T.; Liao, J. C. (2008). "Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels". Nature 451 (7174): 86–9. doi:10.1038/nature06450. PMID 18172501.
- ↑ 16 ECETOC JACC No. 41 n-Butanol (CAS No. 71-36-3), European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, December 2003, pages 3-4.
- ↑ n-Butanol
- ↑ Antoni, D, Zverlov, V. & Schwarz, W H. (2007). "Biofuels from Microbes". Applied Microbiology and Biotechnology 77: 23–35. doi:10.1007/s00253-007-1163-x.
- ↑ Isobutanol at chemicalland21.com
- ↑ Harris O.; et al. (August 1998). Toxicological Profile for 2-Butoxyethanol and 2-butoxyethanol acetate. U.S. Dept of Health and Human Services.
| ||||||||||||||||||