Bürgi–Dunitz angle

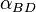 and
and 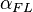 , analogized to altitude- and azimuth-types of parameters in the celestial (horizontal) coordinate system. Both in this celestial coordinate system, and in the system to describe a nucleophile approaching a planar electrophile, the problem is to uniquely describe the location of a point off of a plane, relative to specific point on the plane. Hence, in both cases, the problem can be addressed using two angles, an altitude-type angle and an azimuth-type angle. Note, while elevation in celestial applications is most easily measured as the specific altitude shown, the elevation of the nucleophile,
, analogized to altitude- and azimuth-types of parameters in the celestial (horizontal) coordinate system. Both in this celestial coordinate system, and in the system to describe a nucleophile approaching a planar electrophile, the problem is to uniquely describe the location of a point off of a plane, relative to specific point on the plane. Hence, in both cases, the problem can be addressed using two angles, an altitude-type angle and an azimuth-type angle. Note, while elevation in celestial applications is most easily measured as the specific altitude shown, the elevation of the nucleophile, 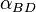 , is most easily measured as the supplementary angle, Nu-C-O, hence its values are most often >90° (see text and next image).
, is most easily measured as the supplementary angle, Nu-C-O, hence its values are most often >90° (see text and next image).
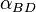 ) angle for this simplest H(–) → H2C=O nucleo-philic addition.
) angle for this simplest H(–) → H2C=O nucleo-philic addition.
 angle in simple systems.
angle in simple systems.

The Bürgi–Dunitz angle (BD angle) is one of two angles that fully define the geometry of "attack" (approach via collision) of a nucleophile on a trigonal unsaturated center in a molecule, originally the carbonyl center in an organic ketone, but now extending to aldehyde, ester, and amide carbonyls, and to alkenes (olefins) as well.[1][2][3] Precisely, in the case of nucleophilic attack at a carbonyl, it is defined as the Nu-C-O bond angle, where Nu is the atom of the nucleophile forming the bond with the carbon. The angle was named after crystallographers Hans-Beat Bürgi and Jack D. Dunitz, its first senior investigators. The second angle defining the geometry describes the "offset" of the nucleophile's approach toward one of the two substituents attached to the carbonyl carbon or other electrophilic center, and was named the Flippin–Lodge angle by Clayton H. Heathcock after his contributing collaborators Lee A. Flippin and Eric P. Lodge.[4] These angles are generally best construed to mean the angle observed/measured for a given system, and not the historically observed value range for the original Bürgi–Dunitz aminoketones, or an idealized value computed for a particular system (such as hydride addition to formaldehyde, image at left). I.e., the BD and FL angles of the hydride-formadehyde system have one pair of values, while the angles observed for other systems are expected to vary.[1][3][5]
The BD angle adopted during an approach by a nucleophile to a trigonal unsaturated electrophile depends primarily on the molecular orbital (MO) shapes and occupancies of the unsaturated center (e.g., carbonyl center), and only secondarily on the molecular orbitals of the nucleophile.[1] Original measurements for a series of intramolecular amine-carbonyl ketone interactions observed in crystals of compounds bearing both functionalities—e.g., methadone and protopine, images at left and right, below—gave a narrow range of BD angle values (105 ± 5°).[2] Corresponding computational estimates (SCF-LCAO-MO calculations) on the approach of the s-orbital of the hydride anion (H−) to the pi-system of the simplest aldehyde, formaldehyde (H2C=O), gave a BD angle value of 107°.[2] Both the crystallographic measurement for aminoketones and the computational estimated for this simplest system are quite close to the theoretical ideal of a tetrahedral angle (internal angles of a tetrahedron, 109.5°), consistent with the importance of this geometry in developing transition states in nucleophilic attacks at trigonal centers.
The convergence of observed BD angles can be viewed as arising from the need to maximize overlap between the highest occupied MO (HOMO) of the nucleophile, and the lowest unoccupied MO (LUMO) of the unsaturated, trigonal center of the electrophile.[1] (See, in comparison, the related inorganic chemistry concept of the angular overlap model.[6][7][8]) In the case of addition to a carbonyl, the HOMO is often a p-type orbital as shown in the figure (e.g., on an amine nitrogen or halide anion), and the LUMO is generally understood to be the antibonding π* MO perpendicular to the plane containing the ketone C=O bond and its substituents (see figure at right above). The BD angle observed for nucleophilic attack is believed to approach the angle that would produce optimal overlap between HOMO and LUMO (based on the principle of the lowering of resulting new MO energies after such mixing of orbitals of similar energy and symmetry from the participating reactants). At the same time, the nucleophile avoids overlap with other orbitals of the electrophilic group that are unfavorable for bond formation (not apparent in image at right, above, because of the simplicity of the R=R'=H in formaldehyde).
To understand cases of real chemical reactions, this HOMO-LUMO centered view has to be modified by an understanding of further complex, electrophile-specific repulsive and attractive electrostatic and van der Waals interactions that alter the BD angle, and bias the Flippin-Lodge angle toward one substituent or the other.[9] In addition, any dynamics at play in the system (e.g., easily change torsional angles) have to be taken into account in real cases. (Recall that BD angle theory was developed based on "frozen" interactions in crystals, while most chemistry takes place via collisions of molecules tumbling in solution.) Moreover, it appears likely that in constrained environments (e.g., in enzyme and nanomaterial binding sites) the BD angles for reactivity will be quite distinct, since normal orbital overlap principles assuming reactivity dependent on random collision are not applicable in simple fashion.[10] For instance, the BD value determined for enzymatic cleavage of an amide by the serine protease subtilisin was 88° (quite distinct from the hydride-formaldehyde value of 107°), and a careful compilation of literature crystallographic BD angle values clustered at 89 ± 7° for the same reaction mediated by different catalysts (i.e., only slightly offset from directly above or below the carbonyl carbon). At the same time, the subtilisin FL value was 8°, see the Flippin-Lodge angle article, and FL angle values from the careful compilation clustered at 4 ± 6° (i.e., only slightly offset from directly behind the carbonyl).[5]
Finally, it is noteworthy that the Bürgi-Dunitz and Flippin-Lodge angles were central, practically, to the development of understanding of asymmetric induction during nucleophilic attack at hindered carbonyl centers (see the Cram–Felkin–Anh and Nguyen model).[4][11] As well, the stereoelectronic principles that underlie nucleophiles adopting a proscribed range of Bürgi–Dunitz angles appears to contribute to the conformational stability of proteins[12][13] and are invoked to explain the stability of particular conformations of molecules in one hypothesis of a chemical origin of life.[14]
References
- 1 2 3 4 Fleming, Ian (2010) Molecular Orbitals and Organic Chemical Reactions: Reference Edition, John Wiley and Sons, pp. 214–215.
- 1 2 3 H. B. Bürgi, J. D. Dunitz, J. M. Lehn, G. Wipff (1974). "Stereochemistry of reaction paths at carbonyl centres". Tetrahedron 30 (12): 1563–1572. doi:10.1016/S0040-4020(01)90678-7.
- 1 2 A.S. Cieplak (2008) Organic addition and elimination reactions: Transformation paths of carbonyl derivatives In Structure Correlation, Vol. 1 (H.-B. Bürgi & J.D. Dunitz, eds.), New York:John Wiley & Sons, pp. 205 - 302, esp. 216-218. [doi:10.1002/9783527616091.ch06; ISBN 9783527616091 ]
- 1 2 C.H. Heathcock (1990) Understanding and controlling diastereofacial selectivity in carbon-carbon bond-forming reactions, Aldrichimica Acta 23(4):94-111, esp. p. 101, see , accessed 9 June 2014.
- 1 2 E.S. Radisky & D.E. Koshland (2002), A clogged gutter mechanism for protease inhibitors, Proc. Natl. Acad. Sci. U.S.A., 99(16):10316-10321, see , accessed 28 November 2014.
- ↑ P.E. Hoggard (2004) Angular overlap model parameters, Struct. Bond. 106, 37.
- ↑ K.F. Purcell & J.C. Kotz (1979) Inorganic Chemistry, Philadelphia, PA:Saunders Company.
- ↑ J.K. Burdett (1978) A new look at structure and bonding in transition metal complexes, Adv. Inorg. Chem. 21, 113.
- ↑ E.P. Lodge & C.H. Heathcock (1987) Steric effects, as well as sigma*-orbital energies, are important in diastereoface differentiation in additions to chiral aldehydes, J. Am. Chem. Soc., 109:3353-3361.
- ↑ S.H. Light, G. Minasov, M.-E. Duban & W.F. Anderson (2014), Adherence to Bürgi-Dunitz stereochemical principles requires significant structural rearrangements in Schiff-base formation: insights from transaldolase complexes, Acta Crystallogr. D Biol. Crystallogr. 70(Pt 2):544-52, DOI: 10.1107/S1399004713030666, see , accessed 10 June 2014.
- ↑ R.E. Gawley & J. Aube, 1996, Principles of Asymmetric Synthesis (Tetrahedron Organic Chemistry Series, Vo. 14), pp. 121-130, esp. pp. 127f.
- ↑ G. J. Bartlett, A. Choudhary, R. T. Raines, D. N. Woolfson (2010). "n→π* interactions in proteins". Nat. Chem. Biol. 6 (8): 615–620. doi:10.1038/nchembio.406. PMC 2921280. PMID 20622857.
- ↑ C. Fufezan (2010). "The role of Buergi‐Dunitz interactions in the structural stability of proteins". Proteins 78 (13): 2831–2838. doi:10.1002/prot.22800. PMID 20635415.
- ↑ A. Choudhary, K. J. Kamer, M. W. Powner, J. D. Sutherland, R. T. Raines (2010). "A stereoelectronic effect in prebiotic nucleotide synthesis". ACS Chem. Biol. 5 (7): 655–657. doi:10.1021/cb100093g. PMC 2912435. PMID 20499895.