Esmolol
 | |
| Systematic (IUPAC) name | |
|---|---|
|
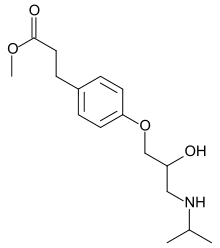
methyl (RS)-3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoate | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| Pregnancy category | |
| Routes of administration | iv |
| Pharmacokinetic data | |
| Bioavailability | - |
| Protein binding | 60% |
| Metabolism | Erythrocytic |
| Biological half-life | 9 minutes |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
103598-03-4 |
| ATC code | C07AB09 |
| PubChem | CID 59768 |
| IUPHAR/BPS | 7178 |
| DrugBank |
DB00187 |
| ChemSpider |
53916 |
| UNII |
MDY902UXSR |
| KEGG |
D07916 |
| ChEBI |
CHEBI:4856 |
| ChEMBL |
CHEMBL768 |
| Chemical data | |
| Formula | C16H25NO4 |
| Molar mass | 295.374 g/mol |
| |
| |
| | |
Esmolol (trade name Brevibloc) is a cardioselective beta1 receptor blocker with rapid onset,[1] a very short duration of action, and no significant intrinsic sympathomimetic or membrane stabilising activity at therapeutic dosages.
It is a class II antiarrhythmic.[2] Esmolol decreases the force and rate of heart contractions by blocking beta-adrenergic receptors of the sympathetic nervous system, which are found in the heart and other organs of the body. Esmolol prevents the action of two naturally occurring substances: epinephrine and norepinephrine.
Dosing
Esmolol is given by slow intravenous injection. It is commonly used in patients during surgery to prevent or treat tachycardia, and is also used in treatment of acute supraventricular tachycardia. Esmolol is also the drug of choice when aortic dissection is suspected.
Metabolism
Esmolol is rapidly metabolized by hydrolysis of the ester linkage, chiefly by the esterases in the cytosol of red blood cells and not by plasma cholinesterases or red cell membrane acetylcholinesterase. Total body clearance in man was found to be about 20 L/kg/hr, which is greater than cardiac output; thus the metabolism of esmolol is not limited by the rate of blood flow to metabolizing tissues such as the liver or affected by hepatic or renal blood flow. Esmolol's short duration of action is based on the ester-methyl side chain which allows for quick hydrolysis. Esmolol's structure is reflected in its name, es-molol as in ester-methyl. Plasma cholinesterases and red cell membrane acetylcholinesterase do not have any action. This metabolism results in the formation of a free acid and methanol. The amount of methanol produced is similar to endogenous methanol production. Esmolol has a rapid distribution half-life of about 2 minutes and an elimination half-life of about 9 minutes.
References
- ↑ Deng CY; Lin SG; Zhang WC; et al. (December 2006). "Esmolol inhibits Na+ current in rat ventricular myocytes". Methods Find Exp Clin Pharmacol 28 (10): 697–702. doi:10.1358/mf.2006.28.10.1037498. PMID 17235414.
- ↑ Jaillon P, Drici M (December 1989). "Recent antiarrhythmic drugs". Am. J. Cardiol. 64 (20): 65J–69J. doi:10.1016/0002-9149(89)91203-4. PMID 2688391.
3. http://www.rxlist.com/brevibloc-drug/clinical-pharmacology.htm accessed Jan 29, 2016.
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||