Bretylium
 | |
| Systematic (IUPAC) name | |
|---|---|
|
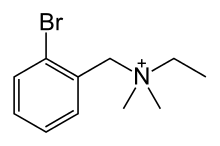
N-(2-bromobenzyl)-N,N-dimethylethanaminium | |
| Clinical data | |
| MedlinePlus | a682861 |
| Pregnancy category | |
| Legal status |
|
| Routes of administration | IV, IM |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | NA |
| Metabolism | None |
| Biological half-life | 7-8 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
59-41-6 |
| ATC code | C01BD02 |
| PubChem | CID 2431 |
| IUPHAR/BPS | 7130 |
| DrugBank |
DB01158 |
| ChemSpider |
2337 |
| UNII |
RZR75EQ2KJ |
| KEGG |
D00645 |
| ChEBI |
CHEBI:3172 |
| ChEMBL |
CHEMBL1199080 |
| Chemical data | |
| Formula | C11H17BrN+ |
| Molar mass | 243.163 g/mol |
| |
| |
| | |
Bretylium (also bretylium tosylate) is an antiarrhythmic agent.[1] It blocks the release of noradrenaline from nerve terminals. In effect, it decreases output from the peripheral sympathetic nervous system. It also acts by blocking K+ channels and is considered a class III antiarrhythmic. The dose is 5–10 mg/kg and side effects are high blood pressure followed by low blood pressure and ventricular ectopy.
Originally introduced in 1959 for the treatment of hypertension.[2] Its use as an antiarrhythmic for ventricular fibrilation was discovered and patented by Marvin Bacaner in 1969 at the University of Minnesota.[3]
The American Heart Association removed Bretylium from their 2000 ECC/ACC guidelines due to its unproven efficacy and ongoing supply problems. Many have cited these supply problems as an issue of raw materials needed in the production of Bretylium. By the release of the AHA 2005 ECC/ACC guidelines there is no mention of Bretylium and it is virtually unavailable throughout most of the world.[4][5]
As of June 8, 2011 Bretylium Tosylate is permanently no longer available in the US after request of Hospira Inc. to withdraw its NDA from the market. Bretylium will remain on the FDA's discontinued drug list since its withdrawal was not the result of a safety or effectiveness concern.[6]
Uses
It was used in emergency medicine, cardiology, and other specialties throughout the 1980s-1990s for the acute management of ventricular tachycardia and ventricular fibrillation refractory to other first line treatments such as defibrillation or lidocaine.[7]
It is contraindicated in patients with AV (atrioventricular) heart block or digoxin toxicity.
Bretylium should be used only in an ICU or Emergency Department setting and should not be used elsewhere due to its dramatic actions and its predominant side effect of hypotension.
Experimental Uses
It is used in physiological and pharmacological research as an inhibitor of sympathetic transmission. Its mechanism of action is the inhibition of neurotransmitter release from sympathetic nerve terminals, both by the inhibition of action potentials in the nerve terminals and by other mechanisms.[8] Its specificity for sympathetic nerves is achieved because it is a substrate for the noradrenaline transporter;[9] hence, it accumulates inside nerve terminals which have this transporter.
Synthesis

Quaternization of o-bromo-N,N-dimethylbenzylamine with ethyl-p-toluenesulfonate yields bretylium sulfonate.
References
- ↑ Tiku, Patience E.; Nowell, Peter T. (1991). "Selective inhibition of K+-stimulation of Na,K-ATPase by bretylium". British Journal of Pharmacology 104 (4): 895–900. doi:10.1111/j.1476-5381.1991.tb12523.x. PMC 1908819. PMID 1667290.
- ↑ Harington, M (1962). "The drug treatment of hypertension. The results of drug treatment". Proceedings of the Royal Society of Medicine 55: 283–6. PMC 1896727. PMID 13904707.
- ↑ "patent". Retrieved 2014-02-06.
- ↑ Khan, M. Gabriel (December 14, 2005). Encyclopedia of Heart Diseases. Academic Press. p. 221. ISBN 978-0-12-406061-6. Retrieved 2015-07-01.
- ↑ Hypothermia~treatment at eMedicine
- ↑ https://www.federalregister.gov/articles/2011/12/19/2011-32367/determination-that-bretylium-tosylate-injection-50-milligramsmilliliter-was-not-withdrawn-from-sale[]
- ↑ "ACS". Archived from the original on September 4, 2006. Retrieved 2008-09-23.
- ↑ Brain, K L; Cunnane, T C (2008). "Bretylium abolishes neurotransmitter release without necessarily abolishing the nerve terminal action potential in sympathetic terminals". British Journal of Pharmacology 153 (4): 831–9. doi:10.1038/sj.bjp.0707623. PMC 2259200. PMID 18071295.
- ↑ Boura, A. L. A.; Copp, F. C.; Duncombe, W. G.; Green, A. F.; McCoubrey, A. (1960). "The selective accumulation of bretylium in sympathetic ganglia and their postganglionic nerves". British Journal of Pharmacology and Chemotherapy 15: 265–70. doi:10.1111/j.1476-5381.1960.tb01242.x. PMC 1481934. PMID 13803289.
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||