Terbutaline
 | |
|
Terbutaline (top), and (R)-(−)-terbutaline (bottom) | |
| Systematic (IUPAC) name | |
|---|---|
|
(RS)-5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diol | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682144 |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral (tablets, oral solution), inhalational (DPI, nebulizer solution), SQ |
| Pharmacokinetic data | |
| Protein binding | 25% |
| Metabolism | GI tract (oral), liver; CYP450: unknown |
| Biological half-life | 11-16 hours |
| Excretion | urine 90% (60% unchanged), bile/faeces |
| Identifiers | |
| CAS Number |
23031-25-6 |
| ATC code | R03AC03 R03CC03 |
| PubChem | CID 5403 |
| IUPHAR/BPS | 560 |
| DrugBank |
DB00871 |
| ChemSpider |
5210 |
| UNII |
N8ONU3L3PG |
| KEGG |
D08570 |
| ChEBI |
CHEBI:9449 |
| ChEMBL |
CHEMBL1760 |
| Chemical data | |
| Formula | C12H19NO3 |
| Molar mass | 225.284 g/mol |
| Chirality | 1 : 1 mixture (racemate) |
| |
| |
| | |
Terbutaline (trade names Brethine, Bricanyl, Brethaire, or Terbulin) is a β2 adrenergic receptor agonist, used as a "reliever" inhaler in the management of asthma symptoms and as a tocolytic (anti-contraction medication) to delay preterm labor for up to 48 hours. This time can then be used to administer steroid injections to the mother which help fetal lung maturity and reduce complications of prematurity.[1] It should not be used to prevent preterm labor or delay labor more than 48–72 hours. In February 2011, the Food and Drug Administration has ordered to put a boxed warning on the drug's label. Pregnant women should not be given injections of the drug terbutaline for the prevention of preterm labor or for long-term (beyond 48–72 hours) management of preterm labor, and should not be given oral terbutaline for any type of prevention or treatment of preterm labor "due to the potential for serious internal heart problems and death."[2][3]
The American College of Obstetricians and Gynecologists also discourages the use of terbutaline for preventing preterm labor.
Terbutaline is currently on the World Anti-Doping Agency's list of prohibited drugs for Olympic athletes, except when administered by inhalation and a Therapeutic Use Exemption (TUE) has been obtained in advance.
Uses
Terbutaline is used as a fast-acting bronchodilator (often used as a short-term asthma treatment) and as a tocolytic[4] to delay premature labor. The inhaled form of terbutaline starts working within 15 minutes and can last up to 6 hours.
Terbutaline as a treatment for premature labor is an off-label use not approved by the FDA. It is a pregnancy category C medication and is routinely prescribed to stop contractions. After successful intravenous tocolysis, little evidence exists that oral terbutaline is effective.[5] However, following uterine inversion in the third stage of labor. Terbutaline (or either Halothane or magnesium sulfate) can be used to relax the uterus if necessary prior to uterine replacement.
Structure activity relationships
The tertiary butyl group in terbutaline makes it more selective for β2 receptors. Since there is no hydroxy group on position 4 of the benzene ring, the molecule is less susceptible to metabolism by the enzyme catechol-O-methyl transferase.[6]
Side effects
- Adult — tachycardia, anxiety, nervousness, tremors, headache, hyperglycemia, hypokalemia, hypotension and, rarely, pulmonary edema.[7]
- Fetal — tachycardia and hypoglycemia.<ref name+"5 Minute Consult (Original source: UpToDate)"> , 5 Minute Consult (Original Source: UpToDate "Terbutaline: Drug information").</ref>
Chemistry
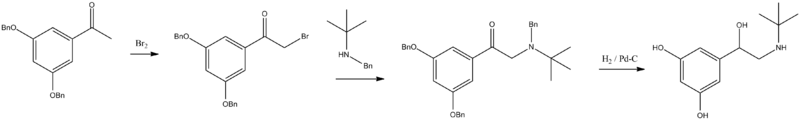
It is synthesized by brominating 3,5-dibenzyloxyacetophenone into the appropriate 3,5-dibenzyloxybromoacetophenone, which is reacted with N-benzyl-N-tert-butylamine, giving the aminoketone. Reduction of this product by hydrogen over a palladium catalyst leads to terbutaline.[8][9][10]
References
- ↑ WHO. "Antenatal administration of corticosteroids for women at risk of preterm birth". WHO. Retrieved 2013-03-25.
- ↑ "Most Popular E-mail Newsletter". USA Today. 18 February 2011.
- ↑ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm243840.htm
- ↑ Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR (September 2008). "Nifedipine versus terbutaline for tocolysis in external cephalic version". Int J Gynaecol Obstet 102 (3): 263–6. doi:10.1016/j.ijgo.2008.04.010. PMID 18554601.
- ↑ Goldenberg, RL (November 2002). "High-Risk Pregnancy Series: An Expert's View". Obstetrics & Gynecology 100 (5): 1020–1037. doi:10.1016/S0029-7844(02)02212-3.
- ↑ Medicinal Chemistry of Adrenergics and Cholinergics
- ↑ Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 7. ISBN 1-59541-101-1.
- ↑ Draco Lunts Farmcetiska Actiebolag, GB 1199630 (1967)
- ↑ Draco Lunts Farmcetiska Actiebolag, BE 704932 (1968)
- ↑ A. L. Swensson, I. K. Weterlin, U.S. Patent 3,937,838 (1976)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||
