Brefeldin A
 | |
| Names | |
|---|---|
| IUPAC name
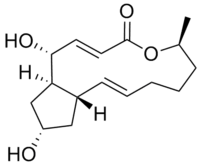
(1R,2E,6S,10E,11aS,13S,14aR)-1,13-Dihydroxy-6-methyl-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f]oxacyclotridecin-4-one | |
| Other names
γ,4-Dihydroxy-2-(6-hydroxy-1-heptenyl)-4-cyclopentanecrotonic acid λ-lactone | |
| Identifiers | |
| 20350-15-6 | |
| ChEBI | CHEBI:48080 |
| ChEMBL | ChEMBL19980 |
| ChemSpider | 4449949 |
| DrugBank | DB07348 |
| Jmol interactive 3D | Image |
| PubChem | 5287620 |
| |
| |
| Properties | |
| C16H24O4 | |
| Molar mass | 280.36 g/mol |
| Appearance | White to off-white crystalline powder |
| Melting point | 204 to 205 °C (399 to 401 °F; 477 to 478 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Brefeldin A is a lactone antibiotic produced by fungal organisms such as Eupenicillium brefeldianum.[1] Brefeldin A inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus indirectly by preventing formation of COPI-mediated[2] transport vesicles. Brefeldin A was initially isolated as an anti-viral antibiotic[3] but is now primarily used in biological research to study protein transport.
Physical data
Brefeldin A forms a clear colorless solution at 10 mg/ml in both dichloromethane and methanol.[4]
Biological effects
In mammalian and yeast cells, the main target of brefeldin A appears to be a Guanine nucleotide exchange factor called GBF1.[5] GBF1 mediates formation of transport vesicles by recruiting COPI coat proteins to cargo-bound receptor proteins found in the membrane of the Golgi. Inhibition of GBF1 activity induced the retrograde movement of secretory proteins from the golgi to the ER. The collapse of the Golgi into the ER triggers activation of unfolded protein response (UPR) (or ER stress)[6][7] which can result in apoptosis.
See also
References
- ↑ Hutchinson, C. R.; Shu-Wen, L.; McInnes, A. G.; Walter, J. A. (1983). "Comparative biochemistry of fatty acid and macrolide antibiotic (brefeldin a). Formation in penicillium brefeldianum". Tetrahedron 39 (21): 3507. doi:10.1016/S0040-4020(01)88660-9.
- ↑ http://www.cellsignal.com/pdf/9972.pdf
- ↑ Tamura G, Ando K, Suzuki S, Takatsuki A, Arima K (February 1968). "Antiviral activity of brefeldin A and verrucarin A". J. Antibiot. 21 (2): 160–1. doi:10.7164/antibiotics.21.160. PMID 4299889.
- ↑ Brefeldin A product page from Fermentek
- ↑ http://www.genecards.org/cgi-bin/carddisp.pl?gene=GBF1
- ↑ Pahl HL, Baeuerle (Jun 1995). "A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B". EMBO J. 14 (11): 2580–8. PMID 7781611.
- ↑ Kober L, Zehe C, Bode J (October 2012). "Development of a novel ER stress based selection system for the isolation of highly productive clones". Biotechnol. Bioeng. 109 (10): 2599–611. doi:10.1002/bit.24527. PMID 22510960.
External links
| Wikimedia Commons has media related to Brefeldin A. |
- Klausner RD, Donaldson JG, Lippincott-Schwartz J (March 1992). "Brefeldin A: insights into the control of membrane traffic and organelle structure". J. Cell Biol. 116 (5): 1071–80. doi:10.1083/jcb.116.5.1071. PMC 2289364. PMID 1740466.
- Nebenführ A, Ritzenthaler C, Robinson DG (November 2002). "Brefeldin A: deciphering an enigmatic inhibitor of secretion". Plant Physiol. 130 (3): 1102–8. doi:10.1104/pp.011569. PMC 1540261. PMID 12427977.
- NCI Frederick, Structure and Data for Brefeldin A. (Image)