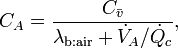Breath gas analysis
Breath gas analysis is a method for gaining non-invasive information on the clinical state of an individual by monitoring volatile organic compounds present in the exhaled breath. Breath gas concentration can then be related to blood concentrations via mathematical modeling as for example in blood alcohol testing.
History
The area of modern breath testing commenced in 1971, when Nobel Prize winner Linus Pauling demonstrated that human breath is a complex gas, containing more than 200 different volatile organic compounds. However, physicians have used breath analysis since the days of Hippocrates.[1]
Overview
Endogenous volatile organic compounds (VOCs) are released within the human organism as a result of normal metabolic activity or due to pathological disorders. They enter the blood stream and are eventually metabolized or excreted via exhalation, skin emission, urine, etc.
Breath sampling is non-invasive and breath samples can be extracted as often as desired.[2]
Identification and quantification of potential disease biomarkers can be seen as the driving force for the analysis of exhaled breath. Moreover, future applications for medical diagnosis and therapy control with dynamic assessments of normal physiological function or pharmacodynamics are intended.
Exogenous VOCs penetrating the body as a result of environmental exposure can be used to quantify body burden. Also breath tests are often based on the ingestion of isotopically labeled precursors, producing isotopically labeled carbon dioxide and potentially many other metabolites.
However, breath sampling is far from being a standardized procedure due to the numerous confounding factors biasing the concentrations of volatiles in breath. These factors are related to both the breath sampling protocols as well as the complex physiological mechanisms underlying pulmonary gas exchange. Even under resting conditions exhaled breath concentrations of VOCs can strongly be influenced by specific physiological parameters such as cardiac output and breathing patterns, depending on the physico-chemical properties of the compound under study.
Understanding the influence of all this factors and their control is necessary for achieving an accurate standardization of breath sample collection and for the correct deduction of the corresponding blood concentration levels.
The simplest model relating breath gas concentration to blood concentrations was developed by Farhi[3]
where  denotes the alveolar concentration which is assumed to be equal to the measured concentration.
It expresses the fact that the concentration of an inert gas in the alveolar air depends on the mixed venous concentration
denotes the alveolar concentration which is assumed to be equal to the measured concentration.
It expresses the fact that the concentration of an inert gas in the alveolar air depends on the mixed venous concentration 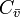 , the substance-specific blood:air partition coefficient
, the substance-specific blood:air partition coefficient 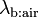 , and the ventilation-perfusion ratio
, and the ventilation-perfusion ratio 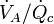 .
But this model fails when two prototypical substances like acetone (partition coefficient
.
But this model fails when two prototypical substances like acetone (partition coefficient 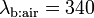 ) or isoprene (partition coefficient
) or isoprene (partition coefficient 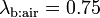 ) are measured.[4]
) are measured.[4]
E.g., multiplying the proposed population mean of approximately 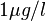 acetone in end-tidal breath by the partition coefficient
acetone in end-tidal breath by the partition coefficient 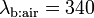 at body temperature grossly underestimates observed (arterial) blood levels spreading around
at body temperature grossly underestimates observed (arterial) blood levels spreading around 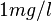 . Furthermore, breath profiles of acetone (and other highly soluble volatile compounds such as 2-pentanone or methyl acetate) associated with moderate workload ergometer challenges of normal healthy volunteers drastically depart from the trend suggested by the equation above.
. Furthermore, breath profiles of acetone (and other highly soluble volatile compounds such as 2-pentanone or methyl acetate) associated with moderate workload ergometer challenges of normal healthy volunteers drastically depart from the trend suggested by the equation above.
Hence some more refined models are necessary. Such models have been developed recently.[5][6]
Applications
Breath gas analysis is used in a number of breath tests.
- Asthma detection by exhaled nitric oxide
- Blood alcohol testing [7]
- Lung cancer detection[8]
- Diabetes detection
- Fructose malabsorption with hydrogen breath test
- Helicobacter pylori with urea breath test
- Diagnosis of bad breath
- Organ rejection
- Carbon Monoxide poisoning
- Smoking cessation
Breath Collectors
Breath can be collected using a variety of home-made and commercially-available devices. The three basic types of breath collector for VOC analysis are:
- Coated stainless steel canister
- End tidal air collector
- Tedlar bag
Each of these can be used as a vehicle for direct introduction of a gas sample into an appropriate analytical instrument, or serve as a reservoir of breath gas into which an absorption device such as an SPME fiber is placed to collect specific compounds.
Analytical instruments
Breath analysis can be done with various forms of mass spectrometry, but there are also simpler methods for specific purposes, such as the Halimeter and the breathalyzer.
- Gas chromatography-mass spectrometry GC-MS
- Proton transfer reaction mass spectrometry PTR-MS and PTR-TOF
- Selected ion flow tube mass spectrometry SIFT-MS
- Ion mobility spectrometry IMS
- Fourier transform infrared spectroscopy FTIR
- Laser spectrometry Spectroscopy
- Chemical sensors resp. Electronic nose
References
- ↑ Anil S. Modak: Single time point diagnostic breath tests: a review, J. Breath Res. 4 (2010), 017002
- ↑ H. Koc, K. Unterkofler, S. Teschl, and J. King: "Mathematical modeling for breath gas analysis," 3. Forschungsforum der Österreichischen Fachhochschulen, Wien 2011.
- ↑ Leon E. Farhi: Elimination of inert gas by the lung, Respiration Physiology 3 (1967) 1–11
- ↑ Julian King, Alexander Kupferthaler, Karl Unterkofler, Helin Koc, Susanne Teschl, Gerald Teschl, Wolfram Miekisch, Jochen Schubert, Hartmann Hinterhuber, and Anton Amann: Isoprene and acetone concentration profiles during exercise at an ergometer, J. Breath Research 3, (2009) 027006 (16 pp)
- ↑ Julian King, Helin Koc, Karl Unterkofler, Pawel Mochalski, Alexander Kupferthaler, Gerald Teschl, Susanne Teschl, Hartmann Hinterhuber, and Anton Amann: Physiological modeling of isoprene dynamics in exhaled breath, J. Theoret. Biol. 267 (2010), 626–637,
- ↑ Julian King, Karl Unterkofler, Gerald Teschl, Susanne Teschl, Helin Koc, Hartmann Hinterhuber, and Anton Amann: A mathematical model for breath gas analysis of volatile organic compounds with special emphasis on acetone, J. Math. Biol. 63 (2011), 959-999,
- ↑ "" Michael P. Hlastala: The alcohol breath test—a review, Journal of Applied Physiology (1998) vol. 84 no. 2, 401–408.
- ↑ "NASA's electronic nose could sniff out cancer", New Scientist, 27 Aug. 2008.
External links
- International Association for Breath Research (IABR)
- Journal of Breath Research
- Computational Breath Analysis project