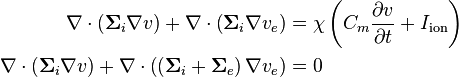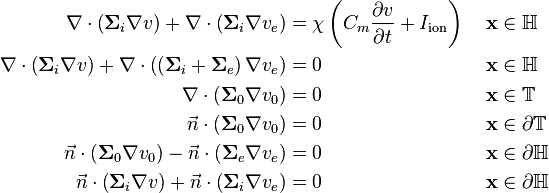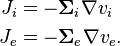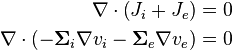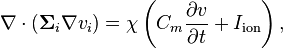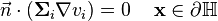Bidomain model
The bidomain model is a mathematical model for the electrical properties of cardiac muscle that takes into account the anisotropy of both the intracellular and extracellular spaces. It is formed of the bidomain equations.
The bidomain model was developed in the late 1970s. [1] [2] [3] [4] [5] [6] [7] [8] It is a generalization of one-dimensional cable theory. The bidomain model is a continuum model, meaning that it represents the average properties of many cells, rather than describing each cell individually. [9]
Many of the interesting properties of the bidomain model arise from the condition of unequal anisotropy ratios. The electrical conductivity in anisotropic tissue is different parallel and perpendicular to the fiber direction. In a tissue with unequal anisotropy ratios, the ratio of conductivities parallel and perpendicular to the fibers is different in the intracellular and extracellular spaces. For instance, in cardiac tissue, the anisotropy ratio in the intracellular space is about 10:1, while in the extracellular space it is about 5:2. [10] Mathematically, unequal anisotropy ratios means that the effect of anisotropy cannot be removed by a change in the distance scale in one direction. [11] Instead, the anisotropy has a more profound influence on the electrical behavior. [12]
Three examples of the impact of unequal anisotropy ratios are
- the distribution of transmembrane potential during unipolar stimulation of a sheet of cardiac tissue,[13]
- the magnetic field produced by an action potential wave front propagating through cardiac tissue,[14]
- the effect of fiber curvature on the transmembrane potential distribution during an electric shock.[15]
The bidomain model is now widely used to model defibrillation of the heart.
Formulation
Standard formulation
The bidomain model can be formulated as follows:
where  is the membrane surface area per unit volume (of tissue),
is the membrane surface area per unit volume (of tissue), 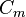 is the electrical capacitance of the membrane per unit area,
is the electrical capacitance of the membrane per unit area, 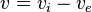 where
where  is the interstitial voltage and
is the interstitial voltage and 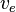 is the extracellular voltage, and
is the extracellular voltage, and 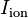 is the ionic current over the membrane per unit area.
is the ionic current over the membrane per unit area.
Formulation with boundary conditions and surrounding tissue
The surrounding tissue 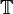 can be included to give reasonable boundary conditions to make the system solvable:
can be included to give reasonable boundary conditions to make the system solvable:
Derivation
Let 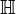 with boundary
with boundary 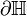 be the set of all points
be the set of all points 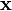 in the heart.
In each point in
in the heart.
In each point in 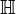 there is an intra- and extracellular voltage and current, denoted by
there is an intra- and extracellular voltage and current, denoted by  ,
, 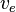 ,
,  and
and  respectively.
Let
respectively.
Let 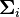 and
and 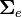 be the intra- end extracellular conductivity tensor matrices respectively.
be the intra- end extracellular conductivity tensor matrices respectively.
We assume Ohmic current-voltage relationship and get
We require that there is no accumulation of charge anywhere in 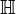 , and therefore that
, and therefore that
giving one of the model equations:
-
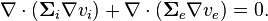
(1)
This equation states that all current exiting one domain must enter the other.
The transmembrane current is given by
-
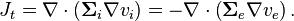
(2)
We model the membrane similarly to that of the cable equation,
-
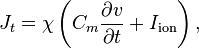
(3)
where  is the membrane surface area per unit volume (of tissue),
is the membrane surface area per unit volume (of tissue), 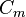 is the electrical capacitance of the membrane per unit area,
is the electrical capacitance of the membrane per unit area, 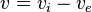 and
and 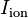 is the ionic current over the membrane per unit area.
is the ionic current over the membrane per unit area.
Combining equations (2) and (3) gives
which can be rearranged using 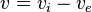 to get another model equation:
to get another model equation:
-
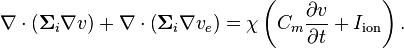
(4)
Boundary conditions
In order to solve the model, boundary conditions are needed. One way to define the boundary condition is to extend the model with a volume 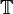 with perimeter
with perimeter 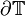 that surrounds the heart and represent the body tissue.
that surrounds the heart and represent the body tissue.
As was the case for 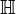 , we assume no accumulation of charge in
, we assume no accumulation of charge in 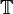 , i.e.
, i.e.
-
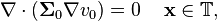
(5)
where 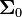 is the conductance tensor of the body tissue and
is the conductance tensor of the body tissue and  is the voltage in
is the voltage in 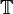 .
.
Assuming that the body is electrically surrounded from the environment, there can be no current component on the surface 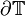 in the normal direction, hence:
in the normal direction, hence:
-
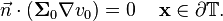
(6)
On the surface of the heart, a common assumption is that there is a direct connection between the surrounding tissue and the extracellular domain. This means that the potentials 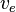 and
and  must be equal on the heart surface, i.e.
must be equal on the heart surface, i.e.
-
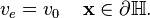
(7)
This direct connection also require that all ionic current exiting 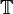 on the heart surface, must enter the extracellular domain, and vica versa. This gives another boundary condition:
on the heart surface, must enter the extracellular domain, and vica versa. This gives another boundary condition:
-
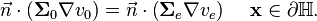
(8)
Finally, we assume that there is a complete isolation of the intracellular domain and the surrounding tissue. Similarly to equation (2), we get
which can be rewritten using 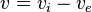 to
to
-
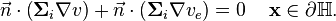
(9)
Extending the model to include equations (5)-(9) gives a solvable system of equations.
Reduction to monodomain model
By assuming equal anisotropy ratios for the intra- and extracellular domains, i.e. 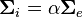 for some scalar
for some scalar  , the model can be reduced to the monodomain model.
, the model can be reduced to the monodomain model.
Numerical solution
There are some special considerations for numerical solution of these equations, due to high time and space resolution needed for numerical convergence.[16] [17]
References
- ↑ Muler AL, Markin VS (1977). "Electrical properties of anisotropic nerve-muscle syncytia-I. Distribution of the electrotonic potential.". Biofizika 22 (2): 307–312. PMID 861269.
- ↑ Muler AL, Markin VS (1977). "Electrical properties of anisotropic nerve-muscle syncytia-II. Spread of flat front of excitation.". Biofizika 22 (3): 518–522. PMID 889914.
- ↑ Muler AL, Markin VS (1977). "Electrical properties of anisotropic nerve-muscle syncytia-III. Steady form of the excitation front.". Biofizika 22 (4): 671–675. PMID 901827.
- ↑ Tung L (1978). "A bi-domain model for describing ischemic myocardial d-c potentials.". PhD dissertation, MIT, Cambridge, Mass.
- ↑ Miller WT III, Geselowitz DB (1978). "Simulation studies of the electrocardiogram, I. The normal heart.". Circulation Research 43 (2): 301–315. doi:10.1161/01.res.43.2.301. PMID 668061.
- ↑ Peskoff A (1979). "Electric potential in three-dimensional electrically syncytial tissues.". Bulletin of Mathematical Biology 41 (2): 163–181. doi:10.1016/s0092-8240(79)80031-2. PMID 760880.
- ↑ Peskoff A (1979). "Electric potential in cylindrical syncytia and muscle fibers.". Bulletin of Mathematical Biology 41 (2): 183–192. doi:10.1016/s0092-8240(79)80032-4. PMID 760881.
- ↑ Eisenberg RS, Barcilon V, Mathias RT (1979). "Electrical properties of spherical syncytia.". Biophysical Journal 48 (3): 449–460. Bibcode:1985BpJ....48..449E. doi:10.1016/S0006-3495(85)83800-5. PMC 1329358. PMID 4041538.
- ↑ Neu JC, Krassowska W (1993). "Homogenization of syncytial tissues.". Critical Reviews of Biomedical Engineering 21: 137–199.
- ↑ Roth BJ (1997). "Electrical conductivity values used with the bidomain model of cardiac tissue.". IEEE Transactions on Biomedical Engineering 44 (4): 326–328. doi:10.1109/10.563303. PMID 9125816.
- ↑ Roth BJ (1992). "How the anisotropy of the intracellular and extracellular conductivities influences stimulation of cardiac muscle.". Journal of Mathematical Biology 30 (6): 633–646. doi:10.1007/BF00948895. PMID 1640183.
- ↑ Henriquez CS (1993). "Simulating the electrical behavior of cardiac tissue using the bidomain model.". Critical Reviews of Biomedical Engineering 21: 1–77.
- ↑ Sepulveda NG, Roth BJ, Wikswo JP Jr (1989). "Current injection into a two-dimensional bidomain.". Biophysical Journal 55 (5): 987–999. Bibcode:1989BpJ....55..987S. doi:10.1016/S0006-3495(89)82897-8. PMC 1330535. PMID 2720084.
- ↑ Sepulveda NG, Wikswo JP Jr (1987). "Electric and magnetic fields from two-dimensional bisyncytia.". Biophysical Journal 51 (4): 557–568. Bibcode:1987BpJ....51..557S. doi:10.1016/S0006-3495(87)83381-7. PMC 1329928. PMID 3580484.
- ↑ Trayanova N, Roth BJ, Malden LJ (1993). "The response of a spherical heart to a uniform electric field: A bidomain analysis of cardiac stimulation.". IEEE Transactions on Biomedical Engineering 40 (9): 899–908. doi:10.1109/10.245611. PMID 8288281.
- ↑ Niederer, S. A.; Kerfoot, E.; Benson, A. P.; Bernabeu, M. O.; Bernus, O.; Bradley, C.; Cherry, E. M.; Clayton, R.; Fenton, F. H.; Garny, A.; Heidenreich, E.; Land, S.; Maleckar, M.; Pathmanathan, P.; Plank, G.; Rodriguez, J. F.; Roy, I.; Sachse, F. B.; Seemann, G.; Skavhaug, O.; Smith, N. P. (3 October 2011). "Verification of cardiac tissue electrophysiology simulators using an N-version benchmark". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369 (1954): 4331–4351. Bibcode:2011RSPTA.369.4331N. doi:10.1098/rsta.2011.0139.
- ↑ Pathmanathan, Pras; Bernabeu, Miguel O.; Bordas, Rafel; Cooper, Jonathan; Garny, Alan; Pitt-Francis, Joe M.; Whiteley, Jonathan P.; Gavaghan, David J. "A numerical guide to the solution of the bidomain equations of cardiac electrophysiology". Progress in Biophysics and Molecular Biology 102 (2-3): 136–155. doi:10.1016/j.pbiomolbio.2010.05.006.