Bicyclononyne
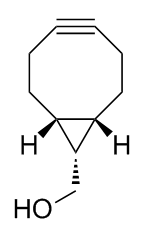
BCN, also known as bicyclo[6.1.0]nonyne, is a copper-free click chemistry probe that enables highly efficient and completely orthogonal bioconjugation to complex macromolecules including peptides, nucleic acids and proteins, including monoclonal antibodies.[1] The most recent and powerful application of this technology has been in the field of antibody-drug conjugates which results in targeted cancer therapeutics that have an improved therapeutic index, meaning they are safer and more effective.[2] Amongst its most notable features, BCN is ideally suited for aqueous bioconjugations due to its high reactivity with its azide counterpart combined with its high hydrophilicity, relative to all other metal-free click chemistry probes.[3] See also "copper-free click chemistry". Commercial use of BCN is proprietary to Netherlands-based biotechnology company, Synaffix BV.[4]
References
- ↑ Dommerholt; et al. (November 2014). "Highly accelerated inverse electron-demand cycloaddition of electron-deficient azides with aliphatic cyclooctynes". Nature Communications. (10)5. doi:10.1038/ncomms6378. PMID 25382411.
- ↑ van Geel; et al. (June 2015). "Chemoenzymatic conjugation of toxic payloads to the globally conserved N-glycan of native mAbs provides homogenous and highly efficacious antibody-drug conjugates". Bioconjugate Chemistry. PMID 26061183.
- ↑ Debets; et al. (September 2011). "Bioconjugation with strained alkenes and alkynes". Acc Chem Res. PMID 21766804.
- ↑ "Metal-Free Click | Synaffix". Synaffix. Retrieved 2015-11-23.