Timolol
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
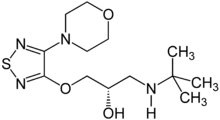
(S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol | |
| Clinical data | |
| Trade names | Timoptic |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a602022 |
| Pregnancy category | |
| Legal status |
|
| Routes of administration | oral, Ophthalmic |
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Metabolism | Hepatic: 80% |
| Biological half-life | 2.5-5 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
26839-75-8 |
| ATC code | C07AA06 S01ED01 |
| PubChem | CID 33624 |
| IUPHAR/BPS | 565 |
| DrugBank |
DB00373 |
| ChemSpider |
31013 |
| UNII |
5JKY92S7BR |
| KEGG |
D08600 |
| ChEBI |
CHEBI:9599 |
| ChEMBL |
CHEMBL499 |
| Chemical data | |
| Formula | C13H24N4O3S |
| Molar mass | 316.421 g/mol |
| |
| |
| (verify) | |
Timolol is a non-selective beta-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headache.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system.[1]
Medical uses
In its by mouth form, it is used:
- to treat high blood pressure
- to prevent heart attacks
- to prevent migraine headaches[2]
In its eye drop form it is used to treat open-angle and occasionally secondary glaucoma by reducing aqueous humour production through blockage of the beta receptors on the ciliary epithelium. The pharmacological mechanism by which it actually does this is still unknown. First beta-blocker approved for topical use in treatment of glaucoma in the USA (1978). With monotherapy, depresses IOP 18-34% below baseline within first few treatments. However, there are short-term escape and long-term drift effects in some patients. That is, tolerance develops. May reduce extent of diurnal IOP curve up to 50%. IOP higher during sleep. 5-10x more potent beta-blocker than propranolol. Light sensitive; preserved with 0.01% benzalkonium Cl (and also comes BAC free). Can also be used in adjunctive therapy with pilocarpine or CAIs.[3]
The 20 January 2014, issue of the Medical Journal of Australia (MJA) reported on topical timolol being successfully used to treat chronic leg ulcers in 92-year-old patient. <1>
A Cochrane Systematic Review compared the effect of timolol versus brimonidine in slowing the progression of open angle glaucoma in adult participants.[4]
Side effects
The most serious possible side effects include cardiac arrhythmias and severe bronchospasms. Timolol can also lead to fainting, congestive heart failure, depression, confusion, worsening of Raynaud's syndrome and impotence.
Side effects when given in the eye include: burning sensation, red eyes, superficial punctate keratopathy, corneal numbness.
Formulations
- Gel-forming solution, ophthalmic, as maleate (Timoptic-XE): 0.25% (2.5 mL, 5 mL); 0.5% (2.5 mL, 5 mL)
- Solution, ophthalmic, as hemihydrate (Betimol): 0.25% (5 mL, 10 mL, 15 mL); 0.5% (5 mL, 10 mL, 15 mL) [contains benzalkonium chloride]
- Solution, ophthalmic, as maleate: 0.25% (5 mL, 10 mL, 15 mL); 0.5% (5 mL, 10 mL, 15 mL) [contains benzalkonium chloride]
- Timoptic: 0.25% (5 mL, 10 mL); 0.5% (5 mL, 10 mL) [contains benzalkonium chloride]
- Solution, ophthalmic, as maleate [preservative free] (Timoptic OcuDose): 0.25% (0.2 mL);0.5% (0.2 mL) [single use]
- Tablet, as maleate (Blocadren): 5 mg, 10 mg, 20 mg
For ophthalmic use, timolol is also available combined with other medications:
- Combigan - timolol and brimonidine
- IOTIM-B - timolol and brimonidine
- Cosopt - timolol maleate and dorzolamide hydrochloride
- DuoTrav - timolol and travoprost
- Xalacom (Pfizer) - timolol and latanoprost
- Brimoeye T ( Preservative free) Brimonidine+Timolol -( Eyekare Kilitch)
- Travaprositn T ( Preservative Free) Travaprost+Timolol- (Eyekare Kilitch)
Brand names
- In Canada: Apo-Timol, Apo-Timop, Gen-Timolol, Nu-Timolol, Phoxal-timolol, PMS-Timolol, Tim-AK, Timoptic, Timoptic-XE.
- In the United States: Betimol, Blocadren, Istalol, Timoptic, Timoptic-XE, Timoptic OcuDose.
- In Jordan: Apimol[5]
- In Israel: V-Optic drops.
- In India: Timoeye, IOTIM.
References
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Dawn A. Marcus; Philip A. Bain (27 February 2009). Effective Migraine Treatment in Pregnant and Lactating Women: A Practical Guide. シュプリンガー・ジャパン株式会社. pp. 141–. ISBN 978-1-60327-438-8. Retrieved 14 November 2010.
- ↑ Strohmaier, K; Snyder, E; Adamsons, I (Jul 1998). "A multicenter study comparing dorzolamide and pilocarpine as adjunctive therapy to timolol: patient preference and impact on daily life". J Am Optom Assoc 69 (7): 441–51. PMID 9697378.
- ↑ Sena DF, Lindsley K (2013). "Neuroprotection for treatment of glaucoma in adults". Cochrane Database Syst Rev 2 (2): CD006539. doi:10.1002/14651858.CD006539.pub3. PMC 3478138. PMID 20166085.
- ↑ http://www.jfda.jo/
External links
- Weinstock, Leonard M.; Mulvey, Dennis M.; Tull, Roger. (1976). "Synthesis of the .beta.-adrenergic blocking agent timolol from optically active precursors". The Journal of Organic Chemistry 41 (19): 3121–3124. doi:10.1021/jo00881a011. PMID 9497.
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||