Beta blocker
| Beta blockers β-blockers | |
|---|---|
| Drug class | |
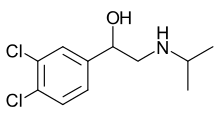
 Skeletal formula of propranolol, the first clinically successful beta blocker | |
| Class identifiers | |
| Use | Hypertension, arrhythmia, etc. |
| ATC code | C07 |
| Biological target | beta receptors |
| Clinical data | |
| AHFS/Drugs.com | Drug Classes |
| Consumer Reports | Best Buy Drugs |
| WebMD | MedicineNet RxList |
| External links | |
| MeSH | D000319 |
Beta blockers (also β-blockers, beta-adrenergic blocking agents, beta antagonists, beta-adrenergic antagonists, beta-adrenoreceptor antagonists, or beta adrenergic receptor antagonists) are a class of drugs that are particularly used for the management of cardiac arrhythmias, protecting the heart from a second heart attack (myocardial infarction) after a first heart attack (secondary prevention).[1] They have been used in hypertension, but are no longer a treatment of first choice.[2][3]
Beta blockers block the action of endogenous catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline) on adrenergic beta receptors, of the sympathetic nervous system, which mediates the fight-or-flight response.[4][5] Some block all activation of β-adrenergic receptors and others are selective.
Three types of beta receptors are known, designated β1, β2 and β3 receptors.[6] β1-adrenergic receptors are located mainly in the heart and in the kidneys.[5] β2-adrenergic receptors are located mainly in the lungs, gastrointestinal tract, liver, uterus, vascular smooth muscle, and skeletal muscle.[5] β3-adrenergic receptors are located in fat cells.[7]
Beta receptors are found on cells of the heart muscles, smooth muscles, airways, arteries, kidneys, and other tissues that are part of the sympathetic nervous system and lead to stress responses, especially when they are stimulated by epinephrine (adrenaline). Beta blockers interfere with the binding to the receptor of epinephrine and other stress hormones, and weaken the effects of stress hormones.
In 1964, Sir James W. Black [8] found the first clinically significant beta blockers—propranolol and pronethalol; it revolutionized the medical management of angina pectoris[9] and is considered by many to be one of the most important contributions to clinical medicine and pharmacology of the 20th century.[10]
For the treatment of primary hypertension, in comparison with other first-line anti-hypertensive drugs, first-line beta-blockers are not as effective in preventing stroke and total cardiovascular events as first-line diuretics, drugs inhibiting the renin-angiotensin system and calcium channel blockers.[11][12][13][14]
Medical uses
Large differences exist in the pharmacology of agents within the class, thus not all beta blockers are used for all indications listed below.
Indications for beta blockers include:
- Angina pectoris[15][16] (contraindicated for Prinzmetal's angina)
- Atrial fibrillation[17]
- Cardiac arrhythmia
- Congestive heart failure
- Essential tremor
- Glaucoma
- Hypertension
- Migraine prophylaxis
- Mitral valve prolapse
- Myocardial infarction
- Phaeochromocytoma, in conjunction with α-blocker
- Postural orthostatic tachycardia syndrome
- Symptomatic control (tachycardia, tremor) in anxiety and hyperthyroidism
- Theophylline overdose
Beta blockers have also been used for:
- Acute aortic dissection
- Hypertrophic obstructive cardiomyopathy
- Marfan syndrome (treatment with propranolol slows progression of aortic dilation and its complications)
- Prevention of variceal bleeding in portal hypertension
- Possible mitigation of hyperhidrosis
- Social and other anxiety disorders
- Controversially, for reduction of perioperative mortality
Congestive heart failure
Although beta blockers were once contraindicated in congestive heart failure, as they have the potential to worsen the condition due to their effect of decreasing cardiac contractility, studies in the late 1990s showed their efficacy at reducing morbidity and mortality.[18][19][20] Bisoprolol, carvedilol, and sustained-release metoprolol are specifically indicated as adjuncts to standard ACE inhibitor and diuretic therapy in congestive heart failure, although at doses typically much lower than what are indicated for other conditions. It should be noted that beta blockers are only indicated in cases of compensated, stable congestive heart failure; in cases of acute decompensated heart failure, beta blockers will cause a further decrease in ejection fraction, worsening the patient's current symptoms.
Beta blockers are known primarily for their reductive effect on heart rate, although this is not the only mechanism of action of importance in congestive heart failure. Beta blockers, in addition to their sympatholytic β1 activity in the heart, influence the renin–angiotensin system at the kidneys. Beta blockers cause a decrease in renin secretion, which in turn reduces the heart oxygen demand by lowering extracellular volume and increasing the oxygen-carrying capacity of blood. Heart failure characteristically involves increased catecholamine activity on the heart, which is responsible for a number of deleterious effects, including increased oxygen demand, propagation of inflammatory mediators, and abnormal cardiac tissue remodeling, all of which decrease the efficiency of cardiac contraction and contribute to the low ejection fraction.[21] Beta blockers counter this inappropriately high sympathetic activity, eventually leading to an improved ejection fraction, despite an initial reduction in ejection fraction.
Trials have shown beta blockers reduce the absolute risk of death by 4.5% over a 13-month period. In addition to reducing the risk of mortality, the numbers of hospital visits and hospitalizations were also reduced in the trials.[22]
Anxiety
Officially, beta blockers are not approved for anxiolytic use by the U.S. Food and Drug Administration.[23] However, many controlled trials in the past 25 years indicate beta blockers are effective in anxiety disorders, though the mechanism of action is not known.[24] The physiological symptoms of the fight-or-flight response (pounding heart, cold/clammy hands, increased respiration, sweating, etc.) are significantly reduced, thus enabling anxious individuals to concentrate on the task at hand.
Musicians, public speakers, actors, and professional dancers have been known to use beta blockers to avoid performance anxiety, stage fright, and tremor during both auditions and public performances. The application to stage fright was first recognized in The Lancet in 1976, and by 1987, a survey conducted by the International Conference of Symphony Orchestra Musicians, representing the 51 largest orchestras in the United States, revealed 27% of its musicians had used beta blockers and 70% obtained them from friends, not physicians.[25] Beta blockers are inexpensive, said to be relatively safe, and on one hand, seem to improve musicians' performances on a technical level, while some, such as Barry Green, the author of "The Inner Game of Music" and Don Greene, a former Olympic diving coach who teaches Juilliard students to overcome their stage fright naturally, say the performances may be perceived as "soulless and inauthentic".[25]
Since they promote lower heart rates and reduce tremors, beta blockers have been used in professional sports where high accuracy is required, including archery, shooting, golf[26] and snooker.[26] Beta blockers are banned by the International Olympic Committee.[27] A recent, high-profile transgression took place in the 2008 Summer Olympics, where 50- metre pistol silver medallist and 10-metre air pistol bronze medallist Kim Jong-su tested positive for propranolol and was stripped of his medals.
For similar reasons, beta blockers have also been used by surgeons.[28]
Surgery
The use of beta blockers around the time of cardiac surgery decreases the risk of heart dysrhythmias.[29] Starting them around the time of other types of surgery, however, worsens outcomes.[29]
Adverse effects
Adverse drug reactions associated with the use of beta blockers include: nausea, diarrhea, bronchospasm, dyspnea, cold extremities, exacerbation of Raynaud's syndrome, bradycardia, hypotension, heart failure, heart block, fatigue, dizziness, alopecia (hair loss), abnormal vision, hallucinations, insomnia, nightmares, sexual dysfunction, erectile dysfunction and/or alteration of glucose and lipid metabolism. Mixed α1/β-antagonist therapy is also commonly associated with orthostatic hypotension. Carvedilol therapy is commonly associated with edema.[30] Due to the high penetration across the blood–brain barrier, lipophilic beta blockers, such as propranolol and metoprolol, are more likely than other, less lipophilic, beta blockers to cause sleep disturbances, such as insomnia, vivid dreams and nightmares.[31]
Adverse effects associated with β2-adrenergic receptor antagonist activity (bronchospasm, peripheral vasoconstriction, alteration of glucose and lipid metabolism) are less common with β1-selective (often termed "cardioselective") agents, but receptor selectivity diminishes at higher doses. Beta blockade, especially of the beta-1 receptor at the macula densa, inhibits renin release, thus decreasing the release of aldosterone. This causes hyponatremia and hyperkalemia.
Hypoglycemia can occur with beta blockade because β2-adrenoceptors normally stimulate hepatic glycogen breakdown (glycogenolysis) and pancreatic release of glucagon, which work together to increase plasma glucose. Therefore, blocking β2-adrenoceptors lowers plasma glucose. β1-blockers have fewer metabolic side effects in diabetic patients; however, the tachycardia that serves as a warning sign for insulin-induced hypoglycemia may be masked. Therefore, beta blockers are to be used cautiously in diabetics.[32]
A 2007 study revealed diuretics and beta blockers used for hypertension increase a patient's risk of developing diabetes, while ACE inhibitors and angiotensin II receptor antagonists (angiotensin receptor blockers) actually decrease the risk of diabetes.[33] Clinical guidelines in Great Britain, but not in the United States, call for avoiding diuretics and beta blockers as first-line treatment of hypertension due to the risk of diabetes.[34]
Beta blockers must not be used in the treatment of cocaine, amphetamine, or other alpha-adrenergic stimulant overdose. The blockade of only beta receptors increases hypertension, reduces coronary blood flow, left ventricular function, and cardiac output and tissue perfusion by means of leaving the alpha-adrenergic system stimulation unopposed. The appropriate antihypertensive drugs to administer during hypertensive crisis resulting from stimulant abuse are vasodilators such as nitroglycerin, diuretics such as furosemide, and alpha blockers such as phentolamine.[35]
Contraindications
Beta blockers are contraindicated in patients with asthma as stated in the British National Formulary 2011. They should also be avoided in patients with a history of cocaine use or in cocaine-induced tachycardia.
Beta blockers should not be used as a first-line treatment in the acute setting for cocaine-induced acute coronary syndrome (CIACS). No recent studies have been identified that show the benefit of beta blockers in reducing coronary vasospasm, or coronary vascular resistance, in patients with CIACS. In the multiple case studies identified, the use of beta blockers in CIACS resulted in detrimental outcomes, and the discontinuation of beta blockers used in the acute setting led to improvement in clinical course. The guidelines by the American College of Cardiology/American Heart Association also support this idea, and recommend against the use of beta blockers in cocaine-induced ST-segment elevation myocardial infarction (MI) because of the risk of coronary vasospasm. Though, in general, beta blockers improve mortality in patients who have suffered MI, it is unclear whether patients with CIACS will benefit from this mortality reduction because no studies assess the use of beta blockers in the long term, and because cocaine users may be prone to continue to abuse the substance, thus complicating the effect of drug therapy.[36]
Toxicity
Glucagon, used in the treatment of overdose,[37][38] increases the strength of heart contractions, increases intracellular cAMP, and decreases renal vascular resistance. It is, therefore, useful in patients with beta-blocker cardiotoxicity.[39][40] Cardiac pacing is usually reserved for patients unresponsive to pharmacological therapy.
Patients experiencing bronchospasm due to the β2 receptor-blocking effects of nonselective beta blockers may be treated with anticholinergic drugs, such as ipratropium, which are safer than beta agonists in patients with cardiovascular disease. Other antidotes for beta-blocker poisoning are salbutamol and isoprenaline.
β-Receptor antagonism
Stimulation of β1 receptors by epinephrine and norepinephrine induces a positive chronotropic and inotropic effect on the heart and increases cardiac conduction velocity and automaticity.[41] Stimulation of β1 receptors on the kidney causes renin release.[42] Stimulation of β2 receptors induces smooth muscle relaxation,[43] induces tremor in skeletal muscle,[44] and increases glycogenolysis in the liver and skeletal muscle.[45] Stimulation of β3 receptors induces lipolysis.[46]
Beta blockers inhibit these normal epinephrine- and norepinephrine-mediated sympathetic actions,[4] but have minimal effect on resting subjects. That is, they reduce excitement/physical exertion on heart rate and force of contraction,[47] and also tremor[48] and breakdown of glycogen, but increase dilation of blood vessels[49] and constriction of bronchi.[50]
Therefore, nonselective beta blockers are expected to have antihypertensive effects.[51] The primary antihypertensive mechanism of beta blockers is unclear, but may involve reduction in cardiac output (due to negative chronotropic and inotropic effects).[52] It may also be due to reduction in renin release from the kidneys, and a central nervous system effect to reduce sympathetic activity (for those beta blockers that do cross the blood–brain barrier, e.g. propranolol).
Antianginal effects result from negative chronotropic and inotropic effects, which decrease cardiac workload and oxygen demand. Negative chronotropic properties of beta blockers allow the lifesaving property of heart rate control. Beta blockers are readily titrated to optimal rate control in many pathologic states.
The antiarrhythmic effects of beta blockers arise from sympathetic nervous system blockade—resulting in depression of sinus node function and atrioventricular node conduction, and prolonged atrial refractory periods. Sotalol, in particular, has additional antiarrhythmic properties and prolongs action potential duration through potassium channel blockade.
Blockade of the sympathetic nervous system on renin release leads to reduced aldosterone via the renin-angiotensin-aldosterone system, with a resultant decrease in blood pressure due to decreased sodium and water retention.
Intrinsic sympathomimetic activity
Also referred to as intrinsic sympathomimetic effect, this term is used particularly with beta blockers that can show both agonism and antagonism at a given beta receptor, depending on the concentration of the agent (beta blocker) and the concentration of the antagonized agent (usually an endogenous compound, such as norepinephrine). See partial agonist for a more general description.
Some beta blockers (e.g. oxprenolol, pindolol, penbutolol, and acebutolol) exhibit intrinsic sympathomimetic activity (ISA). These agents are capable of exerting low-level agonist activity at the β-adrenergic receptor while simultaneously acting as a receptor site antagonist. These agents, therefore, may be useful in individuals exhibiting excessive bradycardia with sustained beta blocker therapy.
Agents with ISA are not used after myocardial infarctions, as they have not been demonstrated to be beneficial. They may also be less effective than other beta blockers in the management of angina and tachyarrhythmia.[30]
α1-Receptor antagonism
Some beta blockers (e.g., labetalol and carvedilol) exhibit mixed antagonism of both β- and α1-adrenergic receptors, which provides additional arteriolar vasodilating action.
Other effects
Beta blockers decrease nocturnal melatonin release, perhaps partly accounting for sleep disturbances caused by some agents.[53]
They can also be used to treat glaucoma because they decrease intraocular pressure by lowering aqueous humor secretion.[54]
Examples
Nonselective agents
- Propranolol
- Bucindolol
- Carteolol
- Carvedilol (has additional α-blocking activity)
- Labetalol (has additional α-blocking activity)
- Nadolol
- Oxprenolol (has intrinsic sympathomimetic activity)
- Penbutolol (has intrinsic sympathomimetic activity)
- Pindolol (has intrinsic sympathomimetic activity)
- Sotalol
- Timolol
- Eucommia bark (herb) [55]
β1-selective agents
Also known as cardioselective
- Acebutolol (has intrinsic sympathomimetic activity)
- Atenolol
- Betaxolol
- Bisoprolol
- Celiprolol
- Esmolol[56]
- Metoprolol
- Nebivolol (also increases nitric oxide release for vasodilation)
β2-selective agents
- Butaxamine (weak α-adrenergic agonist activity): No common clinical applications, but used in experiments
- ICI-118,551: Highly selective β2-adrenergic receptor antagonist—no known clinical applications, but used in experiments due to its strong receptor specificity
β3-selective agents
- SR 59230A (has additional α-blocking activity): Used in experiments
Comparative information
Pharmacological differences
- Agents with intrinsic sympathomimetic action (ISA)
- Acebutolol, carteolol, celiprolol, mepindolol, oxprenolol, pindolol
- Agents with greater aqueous solubility (hydrophilic beta blockers)
- Atenolol, celiprolol, nadolol, sotalol
- Agents with membrane stabilizing effect
- Acebutolol, propranolol
Indication differences
- Agents specifically indicated for cardiac arrhythmia
- Agents specifically indicated for congestive heart failure
- Agents specifically indicated for glaucoma
- Agents specifically indicated for myocardial infarction
- Agents specifically indicated for migraine prophylaxis
Propranolol is the only agent indicated for control of tremor, portal hypertension, and esophageal variceal bleeding, and used in conjunction with α-blocker therapy in phaeochromocytoma.[30]
See also
References
- ↑ Freemantle N, Cleland J, Young P, Mason J, Harrison J (June 1999). "beta Blockade after myocardial infarction: systematic review and meta regression analysis". BMJ 318 (7200): 1730–7. doi:10.1136/bmj.318.7200.1730. PMC 31101. PMID 10381708.
- ↑ Cruickshank JM (August 2010). "Beta blockers in hypertension". Lancet 376 (9739): 415; author reply 415–6. doi:10.1016/S0140-6736(10)61217-2. PMID 20692524.
- ↑ Kaplan, Norman M. (October 2010). "Choice of therapy in primary (essential) hypertension: Clinical trials". UpToDate.
- 1 2 Frishman W.H.; Cheng-Lai A; Nawarskas J (2005). Current Cardiovascular Drugs. Current Science Group. p. 152. ISBN 978-1-57340-221-7. Retrieved 2010-09-07.
- 1 2 3 Arcangelo V.P.; Peterson A.M. (2006). Pharmacotherapeutics for advanced practice: a practical approach. Lippincott Williams & Wilkins. p. 205. ISBN 978-0-7817-5784-3. Retrieved 2010-09-07.
- ↑ Frishman W.H.; Cheng-Lai A; Nawarskas J (2005). Current Cardiovascular Drugs. Current Science Group. p. 153. ISBN 978-1-57340-221-7. Retrieved 2010-09-07.
- ↑ Clément K, Vaisse C, Manning BS, Basdevant A, Guy-Grand B, Ruiz J, Silver KD, Shuldiner AR, Froguel P, Strosberg AD (August 1995). "Genetic variation in the beta 3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity". The New England Journal of Medicine 333 (6): 352–4. doi:10.1056/NEJM199508103330605. PMID 7609752.
- ↑ "Sir James Black inventor of beta-blockers passes away". Retrieved 2010-09-06.
- ↑ van der Vring JA, Daniëls MC, Holwerda NJ, Withagen PJ, Schelling A, Cleophas TJ, Hendriks MG (June 1999). "Combination of calcium channel blockers and beta blockers for patients with exercise-induced angina pectoris: a double-blind parallel-group comparison of different classes of calcium channel blockers. The Netherlands Working Group on Cardiovascular Research (WCN)". Angiology 50 (6): 447–54. doi:10.1177/000331979905000602. PMID 10378820.
- ↑ Stapleton MP (1997). "Sir James Black and propranolol. The role of the basic sciences in the history of cardiovascular pharmacology". Texas Heart Institute Journal 24 (4): 336–42. PMC 325477. PMID 9456487.
- ↑ Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension" Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No.: CD002003. doi:10.1002/14651858.CD002003.pub4
- ↑ Chen JMH, Heran BS, Perez MI, Wright JM. Blood pressure lowering efficacy of beta-blockers as second-line therapy for primary hypertension. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No.: CD007185. DOI: 10.1002/14651858.CD007185.pub2.
- ↑ Xue H, Lu Z, Tang WL, Pang LW, Wang GM, Wong GWK, Wright JM. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension" Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No.: CD008170. doi:10.1002/14651858.CD008170.pub2
- ↑ Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, Wang Y, Yang X, He L. Calcium channel blockers versus other classes of drugs for hypertension" Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No.: CD003654. doi:10.1002/14651858.CD003654.pub4
- ↑ Cleophas, Ton (1995). Beta-blockers in hypertension and angina pectoris: different compounds, different strategies. Kluwer Academic Publishers. ISBN 0-7923-3516-3.
- ↑ Khan, M.I. Gabriel (2007). Cardia Drug Therapy. Humana Press. ISBN 1-59745-238-6.
- ↑ Meinertz T, Willems S (December 2008). "Die Behandlung von Vorhofflimmern im Alltag" [Treatment of atrial fibrillation in every day practice]. Der Internist 49 (12): 1437–42. doi:10.1007/s00108-008-2152-6. PMID 19020848.
- ↑ Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P (2000). "Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group". JAMA 283 (10): 1295–302. doi:10.1001/jama.283.10.1295. PMID 10714728.
- ↑ Leizorovicz A, Lechat P, Cucherat M, Bugnard F (2002). "Bisoprolol for the treatment of chronic heart failure: a meta-analysis on individual data of two placebo-controlled studies--CIBIS and CIBIS II. Cardiac Insufficiency Bisoprolol Study". Am. Heart J. 143 (2): 301–7. doi:10.1067/mhj.2002.120768. PMID 11835035.
- ↑ Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL (2002). "Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study". Circulation 106 (17): 2194–9. doi:10.1161/01.CIR.0000035653.72855.BF. PMID 12390947.
- ↑ "Use of beta blockers and ivabradine in heart failure with reduced ejection fraction". www.uptodate.com. Retrieved 2015-12-11.
- ↑ Pritchett AM, Redfield MM (2002). "Beta-blockers: new standard therapy for heart failure" (PDF). Mayo Clin. Proc. 77 (8): 839–46. doi:10.4065/77.8.839. PMID 12173717.
- ↑ Schneier FR (2006). "Clinical practice. Social anxiety disorder". N. Engl. J. Med. 355 (10): 1029–36. doi:10.1056/NEJMcp060145. PMID 16957148.
- ↑ Tyrer P (1992). "Anxiolytics not acting at the benzodiazepine receptor: Beta blockers". Progress in Neuro-Psychopharmacology and Biological Psychiatry 16 (1): 17–26. doi:10.1016/0278-5846(92)90004-X. PMID 1348368.
- 1 2 Blair Tindall. "Better Playing Through Chemistry", The New York Times, 17 October 2004. Retrieved 3 July 2011.
- 1 2 http://www.independent.co.uk/sport/golf-ogrady-says-players-use-betablockers-drugs-helped-win-majors-1368307.html
- ↑ World Anti-Doping Agency (2005-09-19). "The Worl Anti-Doping Code: The 2006 Prohibited List International Standard" (PDF). World Anti-Doping Agency. Retrieved 2006-12-13.
- ↑ Elman MJ, Sugar J, Fiscella R, Deutsch TA, Noth J, Nyberg M, Packo K, Anderson RJ (1998). "The effect of propranolol versus placebo on resident surgical performance". Transactions of the American Ophthalmological Society 96: 283–91; discussion 291–4. PMC 1298399. PMID 10360293.
- 1 2 Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, Schillinger M, Wiesbauer F, Steinwender C (Sep 18, 2014). "Perioperative beta-blockers for preventing surgery-related mortality and morbidity". The Cochrane database of systematic reviews 9: CD004476. doi:10.1002/14651858.CD004476.pub2. PMID 25233038.
- 1 2 3 Editor Rossi S, ed. (2006). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook.
- ↑ Cruickshank JM (2010). "Beta-blockers and heart failure". Indian Heart J 62 (2): 101–10. PMID 21180298.
- ↑ Beta-Adrenoceptor Antagonists (Beta-Blockers); http://www.cvpharmacology.com/cardioinhibitory/beta-blockers.htm
- ↑ Elliott WJ, Meyer PM (2007). "Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis". Lancet 369 (9557): 201–7. doi:10.1016/S0140-6736(07)60108-1. PMID 17240286.
- ↑ Mayor S (2006). "NICE removes beta blockers as first line treatment for hypertension". BMJ 333 (7557): 8. doi:10.1136/bmj.333.7557.8-a. PMC 1488775. PMID 16809680.
- ↑ eMedicine - Toxicity, Amphetamine : Article by Neal Handly
- ↑ Page RL, Utz KJ, Wolfel EE (December 2007). "Should beta-blockers be used in the treatment of cocaine-associated acute coronary syndrome?". The Annals of Pharmacotherapy 41 (12): 2008–13. doi:10.1345/aph.1H643. PMID 17956961.
- ↑ Weinstein RS, Cole S, Knaster HB, Dahlbert T (February 1985). "Beta blocker overdose with propranolol and with atenolol". Ann Emerg Med 14 (2): 161–3. doi:10.1016/S0196-0644(85)81081-7. PMID 2857542.
- ↑ "Toxicity, Beta-blocker: Treatment & Medication - eMedicine Emergency Medicine". Retrieved 2009-03-06.
- ↑ Beta-Adrenergic Blocker Poisoning; http://www.courses.ahc.umn.edu/pharmacy/6124/handouts/Beta%20blockers.pdf
- ↑ USMLE WORLD 2009 Step1, Pharmacology, Q85
- ↑ Perez, Dianne M. (2006). The Adrenergic Receptors In the 21st Century. Humana Press. p. 135. ISBN 1-58829-423-4. Retrieved 2010-09-08.
- ↑ Jameson, J. Larry; Loscalzo, Joseph (2010). Harrison's Nephrology and Acid-Base Disorders. McGraw-Hill Companies. p. 215. ISBN 0-07-166339-8. Retrieved 2010-09-08.
- ↑ O'Donnell, John M.; Nácul, Flávio E. (2009). Surgical Intensive Care Medicine. Springer. p. 47. ISBN 0-387-77892-6. Retrieved 2010-09-08.
- ↑ Ahrens RC (1990). "Skeletal muscle tremor and the influence of adrenergic drugs". The Journal of Asthma 27 (1): 11–20. doi:10.3109/02770909009073289. PMID 1968452.
- ↑ Reents, Stan (2000). Sport and exercise pharmacology. Human Kinetics. p. 19. ISBN 0-87322-937-1. Retrieved 2010-09-10.
- ↑ Martini, Frederic H. (2005). Anatomy and Physiology. Pearson Education. p. 394. ISBN 0-8053-5947-8. Retrieved 2010-09-10.
- ↑ Khan, M. I. Gabriel (2006). Encyclopedia of Heart Diseases. Elsevier. p. 160. ISBN 978-0-12-406061-6. Retrieved 2010-09-10.
- ↑ Lamster, Ira B.; Northridge, Mary E., eds. (2008). Improving Oral Health for the Elderly: An Interdisciplinary Approach. New York: Springer. p. 87. ISBN 978-0-387-74337-0. Retrieved 2010-10-23.
- ↑ Manger, William Muir; Gifford, Ray Wallace (2001). 100 Questions and Answers about Hypertension. Blackwell Science. p. 106. ISBN 0-632-04481-0. Retrieved 2010-09-10.
- ↑ Rothfeld, Glenn S.; Romaine, Deborah S. (2005). The Encyclopedia of Men's Health. Amaranth. p. 48. ISBN 0-8160-5177-1. Retrieved 2010-10-23.
- ↑ Hurst, J.W. (1997). Schlant, Robert C., ed. Hurst's the Heart 2. Blackwell Science. p. 1564. ISBN 0-07-912951-X. Retrieved 2010-10-07.
- ↑ Reid, J.L. (2001). Lecture notes on clinical pharmacology 6. Blackwell Science. p. 76. ISBN 0-632-05077-2. Retrieved 2011-03-11.
- ↑ Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, Lindner W (1999). "Influence of beta-blockers on melatonin release". Eur. J. Clin. Pharmacol. 55 (2): 111–5. doi:10.1007/s002280050604. PMID 10335905.
- ↑ Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 15. ISBN 1-59541-101-1.
- ↑ Greenway F, Liu Z, Yu Y, Gupta A (2011). "A clinical trial testing the safety and efficacy of a standardized Eucommia ulmoides Oliver bark extract to treat hypertension" (PDF). Alternative medicine review 16 (4): 338–47. PMID 22214253.
- ↑ Umehara S, Goyagi T, Nishikawa T, Tobe Y, Masaki Y (2010). "Esmolol and landiolol, selective beta1-adrenoreceptor antagonists, provide neuroprotection against spinal cord ischemia and reperfusion in rats". Anesthesia and Analgesia 21 (3): 1133–7. doi:10.1213/ANE.0b013e3181cdb06b. PMID 20103544.
External links
- Musicians and beta-blockers by Gerald Klickstein, March 11, 2010 (A blog post that considers "whether beta-blockers are safe, effective, and appropriate for performers to use.")
- Better Playing Through Chemistry by Blair Tindall, New York Times, October 17, 2004. (Discusses the use of beta blockers among professional musicians)
- Musicians using beta blockers by Blair Tindall. Condensed version of above article.
- In Defense of the Beta Blocker by Carl Elliott, The Atlantic, August 20, 2008. (Discusses the use of propranolol by a North Korean pistol shooter in the 2008 Olympics)
- beta-Adrenergic Blockers at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||