Butylone
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
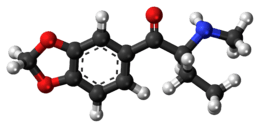
1-(1,3-benzodioxol-5-yl)-2-(methylamino)butan-1-one | |
| Clinical data | |
| Legal status |
|
| Routes of administration | oral, intravenous, insufflation |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 802575-11-7 |
| ATC code | None |
| ChemSpider | 21073070 |
| Chemical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.2524 g/mol |
| |
| |
| Physical data | |
| Density | 1.2 [2] g/cm3 |
| Boiling point | 362.2[2] °C (684.0 °F) |
Butylone, also known as β-keto-N-methylbenzodioxolylbutanamine (bk-MBDB), is an entactogen, psychedelic, and stimulant of the phenethylamine chemical class. It is the β-keto analogue of MBDB. Butylone is produced as a white crystal.[3]
History
Butylone was first synthesized by Koeppe, Ludwig and Zeile which is mentioned in their 1967 paper. It remained an obscure product of academia until 2005 when it was synthesized by a chemical supply company, and has since continued to be sold as a research chemical.[4] It has since been explored as a possible entheogen. Butylone shares the same relationship to MBDB as methylone does to MDMA ("Ecstasy"). The dosage range is not fully understood but seems to be lower than for MBDB. Formal research on this chemical was first conducted in 2009, when it was shown to be metabolised in a similar manner to related drugs like methylone.[5]
Structure and Reactivity
Butylone is a CAD (Cationic amphiphilic drugs), this means that the drug has a hydrophobic section and a cationic hydrophilic section. The hydrophobic section will associate with lipid while the hydrophilic cationic section will associate with the aqueous phase. Lipophilicity of the drug can affect cell membrane permeability. The hydrophobicity allows the drug to interact with membrane receptors. The cationic nature affects movement of Na+ and Ca+ across the cell membrane.
Synthesis
Butylone can be synthesized in a laboratory via the following route: 3,4- methylenedioxybutyrophenone dissolved in dichloromethane to bromine gives 3′,4′-methylenedioxy-2-bromobutyrophenone. This product was then dissolved in dichloromethane and added to an aqueous solution of methylamine (40%). HCl was then added. The aqueous layer was removed and made alkaline by using sodium bicarbonate. For the extraction of the amine ether was used. To get butylone a drop of ether and HCl solution was added. [6]

Metabolism
There are three major metabolic pathways of bk-MBDB as shown in the figure. As result of demethylenation followed by O-methylation bk-MBDB metabolises into 4-OH-3-MeO and 3-OH-4-MeO metabolites in human urine. The second pathway is a β-ketone reduction into β-ketone reduced metabolites. The third pathway is a N-dealkylation into N-dealkyl metabolites. The first two pathways occur more than pathway three. The most common metabolite is the 4-OH-3-MeO metabolite. The metabolites containing a hydroxyl-group would be excreted as their conjugates in urine. [7] [8]

Mechanism of action
Butylone has similar effects as phenethylamines like MDMA and MDBD. It causes an increase in the levels of extracellular serotonin and dopamine. Several studies with animals showed that injections with butylone cause hyperlocomotion. This hyperlocomotion is in direct relationship with the competitive inhibition of dopamine and serotonin uptake in synaptic cells, hereby causing an increase in extracellular levels of dopamine and serotonin. Moreover, butylone activates the 5-HT receptors, which are responsible for extracellular uptake of serotonin and dopamine. When a selective antagonist of the 5-HT receptors as sb-216 641 is added, the effect of butylone is reduced. Butylone is also known for his potency to bind to hDAT, the human dopamine transporter. Furthermore, butylone can bind to VMAT2, which is also known for is dopamine transport abilities. Last butylone has binding activity for the sigma1 receptor, which is responsible for calcium signaling. This is logic cause the calcium concentration has to be regulated for hyperlocomotion. Butylone effects the concentration of dopamine and serotonin, causing hyperlocomotion. Butylone primarily blocks the transporters of serotonin and dopamine and less functions as a substrate for the transporters. [9][10]
Uses
The compound butylone is significantly used as a recreational drug. Reports exist for a different number of methods to consume it,[11] and the risks associated with different routes of administration apply.
Orally
Butylone can be ingested orally, typically with a longer onset and overall duration.
Insufflation
Butylone can be insufflated, with a much quicker onset than oral ingestion.
Intravenous
Butylone can also be administered into the bloodstream directly via intravenous injection, with an instantaneous onset.
Effects
The effects of butylone have not been described in any scientific literature. Some drug forums report personal experiences with butylone. The experiences are similar to methylone and ethylone: euphoria, stimulation, mental sharpness and a warm safe feeling. However the doses planned to be used is exceeded most of the time, the effects last for 10–12 hours [12]
Side Effects
After the effects of this chemical compound disappear some side effects appear: headaches, loss of sleep, depression, and appetite. Not everyone will have all side effects and the side effects will vary per person.[11]
Toxicity
Butylone can cause acute toxicity to specific organs. Single exposure is sufficient for harmful effects. Exposure of butylone also results in skin and eye irritation. Moreover, butylone can cause damage to the respiratory system.[13] Butylone is stable under normal conditions.[14] Formation of toxic gases is possible during heating or in case of fire.
In the following table the half maximal inhibitory concentrations are shown for NET,DAT,SERT respectively norepinephrine, dopamine and serotonin receptors.
| NET IC50 (µM) | 2.02 (1,5-2,7) |
|---|---|
| DAT IC50 (µM) | 2,90 (2,5-3,4) |
| SERT IC50 (µM) | 6,22 (4,3-9,0) |
In the following table the half maximal effective concentrations are shown for DAT and SERT, respectively dopamine and serotonin receptors.
| DAT EC50 (µM) | >100 |
|---|---|
| SERT EC50 (µM) | 5,5 (1,8-17) |
Drug prohibition laws
Sweden
Sveriges riksdag added butylone to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Feb 1, 2010, published by Medical Products Agency in their regulation LVFS 2010:1 listed as Butylon, 1-(1,3-bensodioxol-5-yl)-2-(metylamino)butan-1-on.[16]
United States
Butylone is also a Schedule I substance under the Controlled Substances Act of the United States.
As of October 2015 Butylone is a controlled substance in China.[17]
See also
References
- ↑ = WDU20111050614 "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )" Check
|url= - 1 2 1https://crystalrows.com/pc/butylone
- ↑ http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=NL&language=nl&productNumber=06525&brand=FLUKA&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Ffluka%2F06525%3Flang%3Den
- ↑ Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y. Analysis of designer drugs detected in the products purchased in fiscal year 2006. (Japanese). Yakugaku Zasshi. 2008 Oct;128(10):1499-505. PMID 18827471
- ↑ Zaitsu K, Katagi M, Kamata HT, Kamata T, Shima N, Miki A, Tsuchihashi H, Mori Y (July 2009). "Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine". Forensic Science International 188 (1–3): 131–9. doi:10.1016/j.forsciint.2009.04.001. PMID 19406592.
- ↑ López‐Arnau, R., Martínez‐Clemente, J., Pubill, D., Escubedo, E. and Camarasa, J. (2012), Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. British Journal of Pharmacology, 167: 407–420. doi:10.1111/j.1476-5381.2012.01998.x
- ↑ http://ac.els-cdn.com/S0379073809001558/1-s2.0-S0379073809001558-main.pdf?_tid=edcf700c-c317-11e4-948b-00000aacb35f&acdnat=1425547007_5f64d00dfe02a6528abac4461bf954a2
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3550219/
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3692398/
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3481047/
- 1 2 https://drugs-forum.com/forum/showwiki.php?title=Butylone
- ↑ http://www.soft-tox.org/files/Designer_Drugs/Butylone.pdf
- ↑ http://www.sigmaaldrich.com/catalog/product/fluka/06525?lang=en®ion=NL
- ↑ http://www.lgcstandards.com/WebRoot/Store/Shops/LGC/FilePathPartDocuments/ST-WB-MSDS-1273114-1-1-1.PDF
- 1 2 http://onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2012.02145.x/epdf
- ↑ http://www.lakemedelsverket.se/upload/lvfs/LVFS_2010-1.pdf
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
External links
| ||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||