Epicriptine
Main article: Dihydroergocryptine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
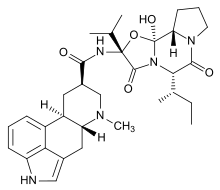
(2R,4R,7R)-N- [(1S,2S,4R,7S)- 2-hydroxy- 7-(S)-(1-methylpropyl)- 5,8-dioxo- 4-(propan-2-yl)- 3-oxa- 6,9-diazatricyclo [7.3.0.02,6]dodecan-4-yl]- 6-methyl- 6,11-diazatetracyclo [7.6.1.02,7.012,16] hexadeca- 1(16),9,12,14-tetraene- 4-carboxamide | |
| Clinical data | |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral |
| Identifiers | |
| CAS Number | 19467-62-0 |
| ATC code | N04BC03 (alpha/beta) |
| PubChem | CID 3084313 |
| ChemSpider | 2341400 |
| UNII | 5M64643B5U |
| ChEBI | CHEBI:59925 |
| ChEMBL | CHEMBL1378681 |
| Synonyms | beta-Dihydroergocryptine |
| Chemical data | |
| Formula | C32H43N5O5 |
| Molar mass | 577.715 g/mol |
| |
| |
Epicriptine or beta-dihydroergocryptine is a dopamine agonist of the ergoline class. It constitutes one third of the mixture known as dihydroergocryptine, the other two thirds consisting of alpha-dihydroergocryptine. The alpha differs from the beta form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid isoleucine is replaced by leucine.[1]
References
| ||||||||||||||||||
This article is issued from Wikipedia - version of the Wednesday, December 16, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.