beta-Alanine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Aminopropanoic acid | |
| Other names
β-Alanine 3-Aminopropionic acid | |
| Identifiers | |
| 107-95-9 | |
| ChEBI | CHEBI:16958 |
| ChEMBL | ChEMBL297569 |
| ChemSpider | 234 |
| DrugBank | DB03107 |
| EC Number | 203-536-5 |
| 2365 | |
| Jmol interactive 3D | Image |
| KEGG | D07561 |
| PubChem | 239 |
| UNII | 11P2JDE17B |
| |
| |
| Properties[1][2] | |
| C3H7NO2 | |
| Molar mass | 89.093 g/mol |
| Appearance | white bipyramidal crystals |
| Odor | odorless |
| Density | 1.437 g/cm3 (19 °C) |
| Melting point | 207 °C (405 °F; 480 K) (decomposes) |
| 54.5 g/100 mL | |
| Solubility | soluble in methanol. diethyl ether, acetone |
| log P | -3.05 |
| Acidity (pKa) | 3.63 |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose) |
1000 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
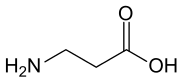
β-Alanine (or beta-alanine) is a naturally occurring beta amino acid, which is an amino acid in which the amino group is at the β-position from the carboxylate group (i.e., two atoms away, see Figure 1). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.
β-Alanine is not used in the biosynthesis of any major proteins or enzymes. It is formed in vivo by the degradation of dihydrouracil and carnosine. It is a component of the naturally occurring peptides carnosine and anserine and also of pantothenic acid (vitamin B5), which itself is a component of coenzyme A. Under normal conditions, β-alanine is metabolized into acetic acid.
β-Alanine is the rate-limiting precursor of carnosine, which is to say carnosine levels are limited by the amount of available β-alanine, not histidine.[3] Supplementation with β-alanine has been shown to increase the concentration of carnosine in muscles, decrease fatigue in athletes and increase total muscular work done.[4][5] Simply supplementing with carnosine is not as effective as supplementing with β-alanine alone since carnosine, when taken orally, is broken down during digestion to its components, histidine and beta-alanine. Hence, by weight, only about 40% of the dose is available as beta-alanine.[3]

Typically, studies have used supplementing strategies of multiple doses of 400 mg or 800 mg, administered at regular intervals for up to eight hours, over periods ranging from 4 to 10 weeks.[5][6] After a 10-week supplementing strategy, the reported increase in intramuscular carnosine content was an average of 80.1% (range 18 to 205%).[5]
L-Histidine, with a pKa of 6.1 is a relatively weak buffer over the physiological intramuscular pH range. However, when bound to other amino acids, this increases nearer to 6.8-7.0. In particular, when bound to β-alanine, the pKa value is 6.83,[7] making this a very efficient intramuscular buffer. Furthermore, because of the position of the beta amino group, β-alanine dipeptides are not incorporated into proteins, and thus can be stored at relatively high concentrations (millimolar). Occurring at 17-25 mmol/kg (dry muscle),[8] carnosine (β-alanyl-L-histidine) is an important intramuscular buffer, constituting 10-20% of the total buffering capacity in type I and II muscle fibres.
Free β-Alanine can cause paraesthesia, a form of neuropathic pain, when ingested in amounts above 10 mg per kilogram of body weight.[6] This is variable between individuals: mild symptoms are experienced by some individuals at 10 mg per kg of body weight; are significant in a majority at 20 mg per kg of body weight; and are severe at 40 mg/kg.[6]
It is probable that the paraesthesia results from high peak blood-plasma concentrations of β-alanine, since an equivalent (equimolar) amount of β-alanine to 40 mg per kg of body weight did not cause paraesthesia when ingested in the form of histidine-containing dipeptides (i.e., carnosine and anserine) in chicken broth extract.[6] In this case, the β-alanine absorption profile is flattened but sustained for a longer period of time,[6] whereas the β-alanine samples in the sports supplementation studies was administered as the free amino acid, resulting in the rapid rise of plasma concentrations, peaking within 30 to 45 minutes, and being eliminated after 90 to 120 minutes. The paraesthesia is not necessary for efficacy, since the published studies undertaken so far have utilized doses of 400 mg or 800 mg at a time to avoid the paraesthesia. Furthermore, excretion of β-alanine in urine accounted for 0.60%(+/-0.09), 1.50%(+/-0.40), or 3.64%(+/-0.47) of the administered doses of 10, 20, or 40 mg per kg body weight,[6] indicating greater losses occurring with increasing dosage.
Even though much weaker than glycine (and, thus, with a debated role as a physiological transmitter), β-alanine is an agonist next in activity to the cognate ligand glycine itself, for strychnine-sensitive inhibitory glycine receptors (GlyRs) (the agonist order: glycine >> β-alanine > taurine >> alanine, L-serine > proline).[9]
A high-potency artificial sweetener, called suosan, is derived from beta-alanine.[10]
References
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 196.
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-83. ISBN 0-8493-0462-8..
- 1 2 http://pharmacistanswers.com/beta-alanine-supplementation-for-exercise-performance.html
- ↑ Derave W, Ozdemir MS, Harris R, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E. (August 9, 2007). "Beta-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters". J Appl Physiol 103 (5): 1736–43. doi:10.1152/japplphysiol.00397.2007. PMID 17690198.
- 1 2 3 Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. (2007). "Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity". Amino Acids 32 (2): 225–33. doi:10.1007/s00726-006-0364-4. PMID 16868650.
- 1 2 3 4 5 6 Harris, RC; Tallon, MJ; Dunnett, M; Boobis, L; Coakley, J; Kim, HJ; Fallowfield, JL; Hill, CA; et al. (2006). "The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis". Amino Acids 30 (3): 279–289. doi:10.1007/s00726-006-0299-9. PMID 16554972.
- ↑ Bate-Smith, EC (1938). "The buffering of muscle in rigor: protein, phosphate and carnosine". Journal of Physiology 92 (3): 336–343. PMC 1395289. PMID 16994977.
- ↑ Mannion, AF; Jakeman, PM; Dunnett, M; Harris, RC; Willan, PLT (1992). "Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans". Eur. J. Appl. Physiol 64: 47–50. doi:10.1007/BF00376439.
- ↑ Encyclopedia of Life Sciences Amino Acid Neurotransmitters. Jeremy M Henley, 2001 John Wiley & Sons, Ltd. doi:10.1038/npg.els.0000010, Article Online Posting Date: April 19, 2001
- ↑ Aspartic acid-beta-4-nitroanilide in the ChemIDplus database
External links
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
