Benzoic anhydride
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Benzoic anhydride | |
| Other names
Benzoic acid anhydride Benzoyl anhydride | |
| Identifiers | |
| 93-97-0 | |
| 516726 | |
| ChemSpider | 6899 |
| EC Number | 202-291-1 |
| Jmol interactive 3D | Image |
| PubChem | 7167 |
| |
| |
| Properties | |
| C 14H 10O 3 | |
| Molar mass | 226.23 g mol−1 |
| Appearance | White to off-white solid |
| Density | 1.1989 g cm−3 at 15 °C |
| Melting point | 42 °C (108 °F; 315 K) |
| Boiling point | 360 °C (680 °F; 633 K) |
| Hazards | |
| NFPA 704 | |
| Flash point | 113[2] °C (235 °F; 386 K) |
| Related compounds | |
| Related compounds |
Benzoic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
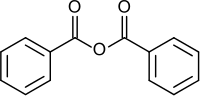
Benzoic anhydride is the acid anhydride of benzoic acid. It is the simplest symmetrical aromatic acid anhydride. It can be produced by dehydrating benzoic acid. It provides a convenient way to prepare benzoic esters, which are more stable to hydrolysis than acetic esters and thus efficient at protecting alcohol function groups in a molecule.[3]
References
- ↑ "Sciencelab msds".
- ↑ "aldrich product page".
- ↑ Organic Synthesis. Academic press. p. 605.
This article is issued from Wikipedia - version of the Saturday, January 02, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
