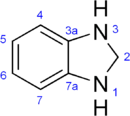
Benzimidazoline
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Dihydro-1H-benzoimidazole | |
| Identifiers | |
| 4746-67-2 | |
| ChemSpider | 10607516 |
| Jmol interactive 3D | Image |
| |
| |
| Properties | |
| C7H8N2 | |
| Molar mass | 120.16 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Benzimidazolines are a family of heterocyclic compounds, based on a benzene ring fused with an imidazoline. The parent compound has the chemical formula C7H8N2.
Benzimidazolines in dihydrogen storage
In one study certain benzimidazolines have been identified as organic hydrides that can be applied in chemical hydrogen storage.[1] Benzimidazoline 1 converts in presence of palladium(II) acetate in acetonitrile at room temperature to the internal salt 2 with formation of hydrogen gas. The salt forms between the benzoate anion and the imidazolium cation where the positive charge is delocalized over 3 atoms. This reaction even takes place in a 3.5 atmospheres of hydrogen. The reaction of benzimidazoline 3 with acetic acid over palladium powder has the same result and 1,3-dimethylbenzimidazoline without a 2-substituent even reacts with water.
This type of reactivity is unusual. Few exergonic reactions exist that liberate hydrogen like the chemical decomposition of formic acid to hydrogen and carbon dioxide. Computations show that the conversion of 1 to 2 and hydrogen is an exergonic reaction with a Gibbs free energy of -21.8 kJ/mol. The formation of (persistent) carbenes from certain imidazolium salts with sodium hydride also generates hydrogen but these reactions are endergonic.

References
- ↑ Schwarz, D. E.; Cameron, T. M.; Hay, P. J.; Scott, B. L.; Tumas, W.; Thorn, D. L. (2005). "Hydrogen Evolution from Organic Hydrides". Chemical Communications 2005 (47): 5919–5921. doi:10.1039/B511884K.