Beetle
| Beetle Temporal range: 318–0 Ma Late Carboniferous–Holocene | |
|---|---|
 | |
| Clockwise from top left: female golden stag beetle (Lamprima aurata), rhinoceros beetle (Megasoma sp.), long nose weevil (Rhinotia hemistictus), cowboy beetle (Chondropyga dorsalis), and a species of Amblytelus. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Hexapoda |
| Class: | Insecta |
| Subclass: | Pterygota |
| Infraclass: | Neoptera |
| Superorder: | Endopterygota |
| Order: | Coleoptera Linnaeus, 1758 |
| Suborders | |
Beetles are a group of insects that form the order Coleoptera /koʊliːˈɒptərə/. The word "coleoptera" is from the Greek κολεός, koleos, meaning "sheath"; and πτερόν, pteron, meaning "wing", thus "sheathed wing", because most beetles have two pairs of wings, the front pair, the "elytra", being hardened and thickened into a shell-like protection for the rear pair and the beetle's abdomen.
Taxonomy and evolution

The Coleopterans include more species than any other order, constituting almost 25% of all known types of animal life forms.[2][3][4] About 450,000 species of beetles occur – representing about 40% of all known insects.[5] Some estimates put the total number of species, described and undescribed, at as high as 100 million, but a figure of one million is more widely accepted.[6] Such a large number of species poses special problems for classification, with some families consisting of thousands of species and needing further division into subfamilies and tribes. This immense number of species allegedly led evolutionary biologist J. B. S. Haldane to quip, when some theologians asked him what could be inferred about the mind of the Creator from the works of His Creation, that God displayed "an inordinate fondness for beetles".[7]
Polyphaga is the largest suborder, containing more than 300,000 described species in more than 170 families, including rove beetles (Staphylinidae), scarab beetles (Scarabaeidae), blister beetles (Meloidae), stag beetles (Lucanidae) and true weevils (Curculionidae). These beetles can be identified by the presence of cervical sclerites (hardened parts of the head used as points of attachment for muscles) absent in the other suborders. The suborder Adephaga contains about 10 families of largely predatory beetles, includes ground beetles (Carabidae), Dytiscidae and whirligig beetles (Gyrinidae). In these beetles, the testes are tubular and the first abdominal sternum (a plate of the exoskeleton) is divided by the hind coxae (the basal joints of the beetle's legs). Archostemata contains four families of mainly wood-eating beetles, including reticulated beetles (Cupedidae) and the telephone-pole beetle. Myxophaga contains about 100 described species in four families, mostly very small, including Hydroscaphidae and the genus Sphaerius.
The oldest known insect that unequivocally resembles species of Coleoptera date back to the Lower Permian (270 mya), though it instead has 13-segmented antennae, elytra with more fully developed venation and more irregular longitudinal ribbing, and an abdomen and ovipositor extending beyond the apex of the elytra. At the end of the Permian, the biggest mass extinction in history took place, collectively called the Permian–Triassic extinction event (P-Tr): 30% of all insect species became extinct; however, it is the only mass extinction of insects in Earth's history until today.[8]
Due to the P-Tr extinction, the fossil record of insects only includes beetles from the Lower Triassic (220 million years ago). Around this time, during the Late Triassic, mycetophagous, or fungus-feeding species (e.g. Cupedidae) appear in the fossil record. In the stages of the Upper Triassic, representatives of the algophagous, or algae-feeding species (e.g. Triaplidae and Hydrophilidae) begin to appear, as well as predatory water beetles. The first primitive weevils appear (e.g. Obrienidae), as well as the first representatives of the rove beetles (e.g. Staphylinidae), which show no marked difference in morphology compared to recent species.[8]
During the Jurassic (210 to 145 million years ago), a dramatic increase in the known diversity of family-level Coleoptera occurred,[8] including the development and growth of carnivorous and herbivorous species. Species of the superfamily Chrysomeloidea are believed to have developed around the same time, which include a wide array of plant hosts ranging from cycads and conifers, to angiosperms.[9] Close to the Upper Jurassic, the portion of the Cupedidae decreased, but at the same time the diversity of the early plant-eating, or phytophagous species increased. Most of the recent phytophagous species of Coleoptera feed on flowering plants or angiosperms. The increase in diversity of the angiosperms is also believed to have influenced the diversity of the phytophagous species, which doubled during the Middle Jurassic. However, doubts have been raised recently, since the increase of the number of beetle families during the Cretaceous does not correlate with the increase of the number of angiosperm species.[10] Also around the same time, numerous primitive weevils (e.g. Curculionoidea) and click beetles (e.g. Elateroidea) appeared. Also, the first jewel beetles (e.g. Buprestidae) are present, but they were rather rare until the Cretaceous.[11][12][13] The first scarab beetles appeared around this time, but they were not coprophagous (feeding upon fecal matter), instead presumably feeding upon the rotting wood with the help of fungus; they are an early example of a mutualistic relationship.
The Cretaceous included the initiation of the most recent round of southern landmass fragmentation, via the opening of the southern Atlantic ocean and the isolation of New Zealand, while South America, Antarctica, and Australia grew more distant.[9] During the Cretaceous, the diversity of Cupedidae and Archostemata decreased considerably. Predatory ground beetles (Carabidae) and rove beetles (Staphylinidae) began to distribute into different patterns; whereas the Carabidae predominantly occurred in the warm regions, the Staphylinidae and click beetles (Elateridae) preferred many areas with temperate climates. Likewise, predatory species of Cleroidea and Cucujoidea hunted their prey under the bark of trees together with the jewel beetles (Buprestidae). The jewel beetles' diversity increased rapidly during the Cretaceous, as they were the primary consumers of wood,[14] while longhorn beetles (Cerambycidae) were rather rare, and their diversity increased only towards the end of the Upper Cretaceous.[8] The first coprophagous beetles have been recorded from the Upper Cretaceous,[15] and are believed to have lived on the excrement of herbivorous dinosaurs, but discussion is still ongoing as to whether the beetles were always tied to mammals during their development.[16] Also, the first species with an adaption of both larvae and adults to the aquatic lifestyle are found. Whirligig beetles (Gyrinidae) were moderately diverse, although other early beetles (e.g. Dytiscidae) were less, with the most widespread being the species of Coptoclavidae, which preyed on aquatic fly larvae.[8]
Between the Paleogene and the Neogene is when today's beetles developed. During this time, the continents began to be located closer to where they are today. Around 5 million years ago, the land bridge between South America and North America was formed, and the fauna exchange between Asia and North America started. Though many recent genera and species already existed during the Miocene, their distribution differed considerably from today's.[8]
Fossil record
A 2007 study based on DNA of living beetles and maps of likely beetle evolution indicated beetles may have originated during the Lower Permian, up to 285 million years ago.[4] In 2009, a fossil beetle was described from the Pennsylvanian of Mazon Creek, Illinois, pushing the origin of the beetles to an earlier date, 318 to 299 million years ago.[17] Fossils from this time have been found in Asia and Europe, for instance in the red slate fossil beds of Niedermoschel near Mainz, Germany.[18] Further fossils have been found in Obora, Czech Republic and Tshekarda in the Ural mountains, Russia.[19] However, there are only a few fossils from North America before the middle Permian, although both Asia and North America had been united to Euramerica. The first discoveries from North America made in the Wellington formation of Oklahoma were published in 2005 and 2008.[8][20]
As a consequence of the Permian–Triassic extinction event, the fossil record of insects is scant, including beetles from the Lower Triassic.[21] However, a few exceptions are noted, as in Eastern Europe; at the Babiy Kamen site in the Kuznetsk Basin, numerous beetle fossils were discovered, even entire specimen of the infraorders Archostemata (e.g. Ademosynidae, Schizocoleidae), Adephaga (e.., Triaplidae, Trachypachidae) and Polyphaga (e.g. Hydrophilidae, Byrrhidae, Elateroidea) and in nearly a perfectly preserved condition.[22] However, species from the families Cupedidae and Schizophoroidae are not present at this site, whereas they dominate at other fossil sites from the Lower Triassic. Further records are known from Khey-Yaga, Russia, in the Korotaikha Basin.[8] There are many important sites from the Jurassic, with more than 150 important sites with beetle fossils, the majority being situated in Eastern Europe and North Asia. In North America and especially in South America and Africa, the number of sites from that time period is smaller, and the sites have not been exhaustively investigated yet. Outstanding fossil sites include Solnhofen in Upper Bavaria, Germany,[23] Karatau in South Kazakhstan,[24] the Yixian formation in Liaoning, North China,[25] as well as the Jiulongshan formation and further fossil sites in Mongolia. In North America there are only a few sites with fossil records of insects from the Jurassic, namely the shell limestone deposits in the Hartford basin, the Deerfield basin and the Newark basin.[8][26]
A large number of important fossil sites worldwide contain beetles from the Cretaceous. Most are located in Europe and Asia and belong to the temperate climate zone during the Cretaceous. A few of the fossil sites mentioned in the chapter Jurassic also shed some light on the early Cretaceous beetle fauna (for example, the Yixian formation in Liaoning, North China).[25] Further important sites from the Lower Cretaceous include the Crato fossil beds in the Araripe basin in the Ceará, North Brazil, as well as overlying Santana formation, with the latter was situated near the paleoequator, or the position of the earth's equator in the geologic past as defined for a specific geologic period. In Spain, important sites are located near Montsec and Las Hoyas. In Australia, the Koonwarra fossil beds of the Korumburra group, South Gippsland, Victoria, are noteworthy. Important fossil sites from the Upper Cretaceous include Kzyl-Dzhar in South Kazakhstan and Arkagala in Russia.[8]
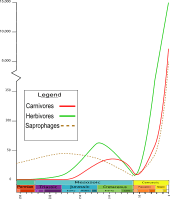
Phylogeny
The superficial consistency of most beetles' morphology, in particular their possession of elytra, has long suggested that Coleoptera is a monophyletic group. Growing evidence indicates this is unjustified, there being arguments for example, in favor of allocating the current suborder Adephaga their own order, or very likely even more than one.[27] The suborders diverged in the Permian and Triassic. Their phylogenetic relationship is uncertain, with the most popular hypothesis being that Polyphaga and Myxophaga are most closely related, with Adephaga as the sister group to those two, and Archostemata as sister to the other three collectively.[9][28] Although six other competing hypotheses are noted, the other most widely discussed one has Myxophaga as the sister group of all remaining beetles rather than just of Polyphaga.[29] Evidence for a close relationship of the two suborders, Polyphaga and Myxophaga, includes the shared reduction in the number of larval leg articles. Adephaga is further considered as sister to Myxophaga and Polyphaga, based on their completely sclerotized elytra, reduced number of crossveins in the hind wings, and the folded (as opposed to rolled) hind wings of those three suborders.
Recent cladistic analysis of some of the structural characteristics supports the Polyphaga and Myxophaga hypothesis.[28] The membership of the clade Coleoptera is not in dispute, with the exception of the twisted-wing parasites, Strepsiptera. These odd insects have been regarded as related to the beetle families Rhipiphoridae and Meloidae, with which they share first-instar larvae that are active, host-seeking triungulins and later-instar larvae that are endoparasites of other insects, or the sister group of beetles, or more distantly related to insects.[29][30] Recent molecular genetic analysis strongly supports the hypothesis that Strepsiptera is the sister group to beetles.[31]
Distribution and diversity
Beetles are by far the largest order of insects, with 350,000–400,000 species in four suborders (Adephaga, Archostemata, Myxophaga, and Polyphaga), making up about 40% of all insect species described, and about 30% of all animals. Though classification at the family level is a bit unstable, about 500 families and subfamilies are recognized.[2][32][33] One of the first proposed estimates of the total number of beetle species on the planet is based on field data rather than on catalog numbers. The technique used for this original estimate, possibly as many as 12 million species, was criticized, and was later revised, with estimates of 850,000–4,000,000 species proposed. Some 70–95% of all beetle species, depending on the estimate, remain undescribed. The beetle fauna is not equally well known in all parts of the world. For example, the known beetle diversity of Australia is estimated at 23,000 species in 3265 genera and 121 families. This is slightly lower than reported for North America, a land mass of similar size with 25,160 species in 3526 genera and 129 families. While other predictions show there could be as many as 28,000 species in North America, including those currently undescribed, a realistic estimate of the little-studied Australian beetle fauna's true diversity could vary from 80,000 to 100,000.[34]
Coleoptera are found in nearly all natural habitats, including freshwater and marine habitats, everywhere vegetative foliage is found, from trees and their bark to flowers, leaves, and underground near roots- even inside plants in galls, in every plant tissue, including dead or decaying ones.[35]
External morphology

Beetles are generally characterized by a particularly hard exoskeleton and hard forewings (elytra). The beetle's exoskeleton is made up of numerous plates, called sclerites, separated by thin sutures. This design provides armored defenses while maintaining flexibility. The general anatomy of a beetle is quite uniform, although specific organs and appendages may vary greatly in appearance and function between the many families in the order. Like all insects, beetles' bodies are divided into three sections: the head, the thorax, and the abdomen.[2]
Head


The head, having mouthparts projecting forward or sometimes downturned, is usually heavily sclerotized and varies in size.[32] The eyes are compound and may display remarkable adaptability, as in the case of whirligig beetles (family Gyrinidae), where they are split to allow a view both above and below the waterline. Other species also have divided eyes – some longhorn beetles (family Cerambycidae) and weevils – while many have eyes that are notched to some degree. A few beetle genera also possess ocelli, which are small, simple eyes usually situated farther back on the head (on the vertex).
Beetles' antennae are primarily organs of smell, but may also be used to feel a beetle's environment physically. They may also be used in some families during mating, or among a few beetle species for defence. Antennae vary greatly in form within the Coleoptera, but are often similar within any given family. Males and females sometimes have different antennal forms. Antennae may be clavate (flabellate and lamellate are subforms of clavate, or clubbed antennae), filiform, geniculate, moniliform, pectinate, or serrate.
Beetles have mouthparts similar to those of grasshoppers. Of these parts, the most commonly known are probably the mandibles, which appear as large pincers on the front of some beetles. The mandibles are a pair of hard, often tooth-like structures that move horizontally to grasp, crush, or cut food or enemies (see defence, below). Two pairs of finger-like appendages, the maxillary and labial palpi, are found around the mouth in most beetles, serving to move food into the mouth. In many species, the mandibles are sexually dimorphic, with the males' enlarged enormously compared with those of females of the same species.[32]
Thorax
The thorax is segmented into the two discernible parts, the pro- and pterathorax. The pterathorax is the fused meso- and metathorax, which are commonly separated in other insect species, although flexibly articulate from the prothorax. When viewed from below, the thorax is that part from which all three pairs of legs and both pairs of wings arise. The abdomen is everything posterior to the thorax.[2] When viewed from above, most beetles appear to have three clear sections, but this is deceptive: on the beetle's upper surface, the middle "section" is a hard plate called the pronotum, which is only the front part of the thorax; the back part of the thorax is concealed by the beetle's wings. This further segmentation is usually best seen on the abdomen.

Extremities
The multisegmented legs end in two to five small segments called tarsi. Like many other insect orders, beetles bear claws, usually one pair, on the end of the last tarsal segment of each leg. While most beetles use their legs for walking, legs may be variously modified and adapted for other uses. Among aquatic families – Dytiscidae, Haliplidae, many species of Hydrophilidae and others – the legs, most notably the last pair, are modified for swimming and often bear rows of long hairs to aid this purpose. Other beetles have fossorial legs that are widened and often spined for digging. Species with such adaptations are found among the scarabs, ground beetles, and clown beetles (family Histeridae). The hind legs of some beetles, such as flea beetles (within Chrysomelidae) and flea weevils (within Curculionidae), are enlarged and designed for jumping.
Wings
The elytra are connected to the pterathorax, so named because it is where the wings are connected (pteron meaning "wing" in Greek).[2] The elytra are not used for flight, but tend to cover the hind part of the body and protect the second pair of wings (alae). They must be raised to move the hind flight wings. A beetle's flight wings are crossed with veins and are folded after landing, often along these veins, and stored below the elytra. A fold (jugum) of the membrane at the base of each wing is a characteristic feature.[36] In some beetles, the ability to fly has been lost. These include some ground beetles (family Carabidae) and some "true weevils" (family Curculionidae), but also desert- and cave-dwelling species of other families. Many have the two elytra fused together, forming a solid shield over the abdomen. In a few families, both the ability to fly and the elytra have been lost, with the best known example being the glow-worms of the family Phengodidae, in which the females are larviform throughout their lives.
Abdomen
The abdomen is the section behind the metathorax, made up of a series of rings, each with a hole for breathing and respiration, called a spiracle, composing three different segmented sclerites: the tergum, pleura, and the sternum. The tergum in almost all species is membranous, or usually soft and concealed by the wings and elytra when not in flight. The pleura are usually small or hidden in some species, with each pleuron having a single spiracle. The sternum is the most widely visible part of the abdomen, being a more or less sclerotized segment. The abdomen itself does not have any appendages, but some (for example, Mordellidae) have articulating sternal lobes.[37]
Internal morphology
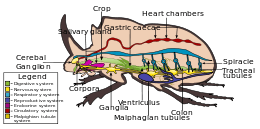
Digestive system
The digestive system of beetles is primarily based on plants, upon which they, for the most part, feed, with mostly the anterior midgut performing digestion, although in predatory species (for example Carabidae), most digestion occurs in the crop by means of midgut enzymes. In Elateridae species, the predatory larvae defecate enzymes on their prey, with digestion being extraorally.[2] The alimentary canal basically consists of a short, narrow pharynx, a widened expansion, the crop, and a poorly developed gizzard. After is the midgut, that varies in dimensions between species, with a large amount of cecum, with a hindgut, with varying lengths. Typically, four to six Malpighian tubules occur.[32]
Nervous system
The nervous system in beetles contains all the types found in insects, varying between different species, from three thoracic and seven or eight abdominal ganglia which can be distinguished to that in which all the thoracic and abdominal ganglia are fused to form a composite structure.[2]
Respiratory system
Like most insects, beetles inhale oxygen and exhale carbon dioxide via a tracheal system. Air enters the body through spiracles, and circulates within the haemocoel in a system of tracheae and tracheoles, through the walls of which the relevant gases can diffuse appropriately.[2]
Diving beetles, such as the Dytiscidae, carry a bubble of air with them when they dive. Such a bubble may be contained under the elytra or against the body by specialized hydrophobic hairs. The bubble covers at least some of the spiracles, thereby permitting the oxygen to enter the tracheae.[2]
The function of the bubble is not so much as to contain a store of air, but to act as a physical gill. The air that it traps is in contact with oxygenated water, so as the animal's consumption depletes the oxygen in the bubble, more oxygen can diffuse in to replenish it. Carbon dioxide is more soluble in water than either oxygen or nitrogen, so it readily diffuses out faster than in. Nitrogen is the most plentiful gas in the bubble, and the least soluble, so it constitutes a relatively static component of the bubble and acts as a stable medium for respiratory gases to accumulate in and pass through. Occasional visits to the surface are sufficient for the beetle to re-establish the constitution of the bubble.[38]
Circulatory system
Like other insects, beetles have open circulatory systems, based on hemolymph rather than blood. Also as in other insects, a segmented tube-like heart is attached to the dorsal wall of the hemocoel. It has paired inlets or ostia at intervals down its length, and circulates the hemolymph from the main cavity of the haemocoel and out through the anterior cavity in the head.
Specialized organs
Different glands specialize for different pheromones produced for finding mates. Pheromones from species of Rutelinea are produced from epithelial cells lining the inner surface of the apical abdominal segments; amino acid-based pheromones of Melolonthinae are produced from eversible glands on the abdominal apex. Other species produce different types of pheromones. Dermestids produce esters, and species of Elateridae produce fatty acid-derived aldehydes and acetates.[2] For means of finding a mate also, fireflies (Lampyridae) use modified fat body cells with transparent surfaces backed with reflective uric acid crystals to biosynthetically produce light, or bioluminescence. The light produce is highly efficient, as it is produced by oxidation of luciferin by enzymes (luciferases) in the presence of adenosine triphosphate (ATP) and oxygen, producing oxyluciferin, carbon dioxide, and light.[2]
A notable number of species have developed special glands to produce chemicals for deterring predators (see Defense and predation). The ground beetle's (of Carabidae) defensive glands, located at the posterior, produce a variety of hydrocarbons, aldehydes, phenols, quinones, esters, and acids released from an opening at the end of the abdomen. African carabid beetles (for example, Anthia and Thermophilum – Thermophilum generally included within Anthia) employ the same chemicals as ants: formic acid.[39] Bombardier beetles have well-developed, like other carabid beetles, pygidial glands that empty from the lateral edges of the intersegment membranes between the seventh and eighth abdominal segments. The gland is made of two containing chambers. The first holds hydroquinones and hydrogen peroxide, with the second holding just hydrogen peroxide plus catalases. These chemicals mix and result in an explosive ejection, forming temperatures of around 100 °C (212 °F), with the breakdown of hydroquinone to H2 + O2 + quinone, with the O2 propelling the excretion.[2]
Tympanal organs or hearing organs, which is a membrane (tympanum) stretched across a frame backed by an air sac and associated sensory neurons, are described in two families.[40] Several species of the genus Cicindela (Cicindelidae) have ears on the dorsal surfaces of their first abdominal segments beneath the wings; two tribes in the subfamily Dynastinae (Scarabaeidae) have ears just beneath their pronotal shields or neck membranes. The ears of both families are sensitive to ultrasonic frequencies, with strong evidence indicating they function to detect the presence of bats by their ultrasonic echolocation. Though beetles constitute a large order and live in a variety of niches, examples of hearing are surprisingly lacking amongst species, though likely most simply remain undiscovered.[2]
Reproduction and development
Beetles are members of the superorder Endopterygota, and accordingly most of them undergo complete metamorphosis. The typical form of metamorphosis in beetles passes through four main stages: the egg, the larva, the pupa, and the imago or adult. The larvae are commonly called grubs and the pupa sometimes is called the chrysalis. In some species, the pupa may be enclosed in a cocoon constructed by the larva towards the end of its final instar. Going beyond "complete metamorphosis", however, some beetles, such as typical members of the families Meloidae and Rhipiphoridae, undergo hypermetamorphosis in which the first instar takes the form of a triungulin.
Mating

Beetles may display extremely intricate behavior when mating. Pheromone communication is likely to be important in the location of a mate.
Different species use different chemicals for their pheromones. Some scarab beetles (for example, Rutelinae) utilize pheromones derived from fatty acid synthesis, while other scarab beetles use amino acids and terpenoid compounds (for example, Melolonthinae). Another way species of Coleoptera find mates is the use of biosynthesized light, or bioluminescence. This special form of a mating call is confined to fireflies (Lampyridae) by the use of abdominal light-producing organs. The males and females engage in complex dialogue before mating, identifying different species by differences in duration, flight patterns, composition, and intensity.[2]
Before mating, males and females may engage in various forms of behavior. They may stridulate, or vibrate the objects they are on. In some species (for example, Meloidae), the male climbs onto the dorsum of the female and strokes his antennae on her head, palps, and antennae. In the genus Eupompha of said family, the male draws the antennae along his longitudinal vertex. They may not mate at all if they do not perform the precopulatory ritual.[2]
Conflict can play a part in the mating rituals of species such as burying beetles (genus Nicrophorus), where conflicts between males and females rage until only one of each is left, thus ensuring reproduction by the strongest and fittest. Many male beetles are territorial and fiercely defend their small patches of territory from intruding males. In such species, the male often has horns on the head or thorax, making its body length greater than that of a female. Pairing is generally quick, but in some cases lasts for several hours. During pairing, sperm cells are transferred to the female to fertilize the egg.[32]
Lifecycle

Egg
A single female may lay from several dozen to several thousand eggs during her lifetime. Eggs are usually laid according to the substrate on which the larvae feed upon hatching. Among others, they can be laid loose in the substrate (for example, flour beetle), laid in clumps on leaves (for example, Colorado potato beetle), individually attached (for example, mungbean beetle and other seed borers), or buried in the medium (for example, carrot weevil).
Parental care varies between species, ranging from the simple laying of eggs under a leaf to certain scarab beetles, which construct underground structures complete with a supply of dung to house and feed their young.[2] Other beetles are leaf rollers, biting sections of leaves to cause them to curl inwards, then laying their eggs, thus protected, inside.[2]
Larva

The larva is usually the principal feeding stage of the beetle lifecycle. Larvae tend to feed voraciously once they emerge from their eggs. Some feed externally on plants, such as those of certain leaf beetles, while others feed within their food sources. Examples of internal feeders are most Buprestidae and longhorn beetles. The larvae of many beetle families are predatory like the adults (ground beetles, ladybirds, rove beetles). The larval period varies between species, but can be as long as several years. The larvae are highly varied amongst species, with well-developed and sclerotized heads, and have distinguishable thoracic and abdominal segments (usually the tenth, though sometimes the eighth or ninth).[32]
Beetle larvae can be differentiated from other insect larvae by their hardened, often darkened heads, the presence of chewing mouthparts, and spiracles along the sides of their bodies. Like adult beetles, the larvae are varied in appearance, particularly between beetle families. Beetles whose larvae are somewhat flattened and are highly mobile are the ground beetles, some rove beetles, and others; their larvae are described as campodeiform. Some beetle larvae resemble hardened worms with dark head capsules and minute legs. These are elateriform larvae, and are found in the click beetle (Elateridae) and darkling beetle (Tenebrionidae) families. Some elateriform larvae of click beetles are known as wireworms. Beetles in the families of the Scarabaeoidea have short, thick larvae described as scarabaeiform, but more commonly known as grubs.
All beetle larvae go through several instars, which are the developmental stages between each moult. In many species, the larvae simply increase in size with each successive instar as more food is consumed. In some cases, however, more dramatic changes occur. Among certain beetle families or genera, particularly those that exhibit parasitic lifestyles, the first instar (the planidium) is highly mobile to search out a host, while the following instars are more sedentary and remain on or within their host. This is known as hypermetamorphosis; examples include the blister beetles (family Meloidae) and some rove beetles, particularly those of the genus Aleochara.
Pupa
As with all endopterygotes, beetle larvae pupate, and from these pupae emerge fully formed, sexually mature adult beetles, or imagos. Adults have extremely variable lifespans, from weeks to years, depending on the species. In some species, the pupa may go through all four forms during its development, called hypermetamorphosis (for example, Meloidae). Pupae always have no mandibles (are adecticous). In most, the appendages are not attached to the pupae; ones that do have appendages are mostly obtect, and the rest are exarate.[32]
Behavior
Locomotion

Aquatic beetles use several techniques for retaining air beneath the water's surface. Beetles of the family Dytiscidae hold air between the abdomen and the elytra when diving. Hydrophilidae have hairs on their under surface that retain a layer of air against their bodies. Adult crawling water beetles use both their elytra and their hind coxae (the basal segment of the back legs) in air retention,[41] while whirligig beetles simply carry an air bubble down with them whenever they dive.
The elytra allow beetles and weevils to both fly and move through confined spaces, doing so by folding the delicate wings under the elytra while not flying, and folding their wings out just before take off. The unfolding and folding of the wings is operated by muscles attached to the wing base; as long as the tension on the radial and cubital veins remains, the wings remain straight. In day-flying species (for example, Buprestidae, Scarabaeidae), flight does not include large amounts of lifting of the elytra, having the metathorac wings extended under the lateral elytra margins.[2]
Communication
Beetles have a variety of ways to communicate, some of which include a sophisticated chemical language through the use of pheromones. From the host tree, mountain pine beetles have many forms of communication. They can emit both an aggregative pheromone and an anti-aggregative pheromone. The aggregative pheromone attracts other beetles to the tree, and the anti-aggregative pheromone neutralizes the aggregative pheromone. This helps to avoid the harmful effects of having too many beetles on one tree competing for resources. The mountain pine beetle can also stridulate to communicate, or rub body parts together to create sound, having a "scraper" on their abdomens that they rub against a grooved surface on the underside of their left wing cover to create a sound that is not audible to humans. Once the female beetles have arrived on a suitable pine tree host, they begin to stridulate and produce aggregative pheromones to attract other unmated males and females. New females arrive and do the same as they land and bore into the tree. As the males arrive, they enter the galleries that the females have tunneled, and begin to stridulate to let the females know they have arrived, and to also warn others that the female in that gallery is taken. At this point, the female stops producing aggregative pheromones and starts producing anti-aggregative pheromone to deter more beetles from coming.[42]
Since species of Coleoptera use environmental stimuli to communicate, they are affected by the climate. Microclimates, such as wind or temperature, can disturb the use of pheromones; wind would blow the pheromones while they travel through the air. Stridulating can be interrupted when the stimulus is vibrated by something else.[42]
Parental care

Among insects, parental care is very uncommon, only found in a few species. Some beetles also display this unique social behavior.[2] One theory states parental care is necessary for the survival of the larvae, protecting them from adverse environmental conditions and predators. One species, a rover beetle (Bledius spectabilis) displays both causes for parental care: physical and biotic environmental factors. Said species lives in salt marshes, so the eggs and/or larvae are endangered by the rising tide. The maternal beetle patrols the eggs and larvae and applies the appropriate burrowing behavior to keep them from flooding and from asphyxiating. Another advantage is that the mother protects the eggs and larvae from the predatory carabid beetle Dicheirotrichus gustavi and from the parasitoid wasp Barycnemis blediator. Up to 15% of larvae are killed by this parasitoid wasp, being only protected by maternal beetles in their dens.[43]
Some species of dung beetle also display a form of parental care. Dung beetles collect animal feces, or "dung", from which their name is derived, and roll it into a ball, sometimes being up to 50 times their own weight; albeit sometimes it is also used to store food. Usually it is the male that rolls the ball, with the female hitch-hiking or simply following behind. In some cases the male and the female roll together. When a spot with soft soil is found, they stop and bury the dung ball. They then mate underground. After the mating, one or both of them prepares the brooding ball. When the ball is finished, the female lays eggs inside it, a form of mass provisioning. Some species do not leave after this stage, but remain to safeguard their offspring.[44]
_on_Ipomoea_carnea_W_IMG_0593.jpg)
Feeding
Besides being abundant and varied, beetles are able to exploit the wide diversity of food sources available in their many habitats. Some are omnivores, eating both plants and animals. Other beetles are highly specialized in their diet. Many species of leaf beetles, longhorn beetles, and weevils are very host-specific, feeding on only a single species of plant. Ground beetles and rove beetles (family Staphylinidae), among others, are primarily carnivorous and catch and consume many other arthropods and small prey, such as earthworms and snails. While most predatory beetles are generalists, a few species have more specific prey requirements or preferences.[45]
Decaying organic matter is a primary diet for many species. This can range from dung, which is consumed by coprophagous species (such as certain scarab beetles of the family Scarabaeidae), to dead animals, which are eaten by necrophagous species (such as the carrion beetles of the family Silphidae). Some of the beetles found within dung and carrion are in fact predatory. These include the clown beetles, preying on the larvae of coprophagous and necrophagous insects.
Ecology
Defense and predation

Beetles and their larvae have a variety of strategies to avoid being attacked by predators or parasitoids. These include camouflage, mimicry, toxicity, and active defense. Camouflage involves the use of coloration or shape to blend into the surrounding environment. This sort of protective coloration is common and widespread among beetle families, especially those that feed on wood or vegetation, such as many of the leaf beetles (family Chrysomelidae) or weevils. In some of these species, sculpturing or various colored scales or hairs cause the beetle to resemble bird dung or other inedible objects. Many of those that live in sandy environments blend in with the coloration of the substrate.[46] The giant African longhorn beetle (Petrognatha gigas) resembles the moss and bark of the tree it feeds on. Another defense that often uses color or shape to deceive potential enemies is mimicry. Some longhorn beetles (family Cerambycidae) bear a striking resemblance to wasps, which helps them avoid predation even though the beetles are in fact harmless. This defense is an example of Batesian mimicry and, together with other forms of mimicry and camouflage occurs widely in other beetle families, such as the Scarabaeidae. Beetles may combine their color mimicry with behavioral mimicry, acting like the wasps they already closely resemble. Many beetle species, including ladybirds, blister beetles, and lycid beetles can secrete distasteful or toxic substances to make them unpalatable or even poisonous. These same species are often aposematic, where bright or contrasting color patterns warn away potential predators; many beetles and other insects mimic these chemically protected species.[39]

Chemical defense is another important defense found amongst species of Coleoptera, usually being advertised by bright colors. Others may utilize behaviors that would be done when releasing noxious chemicals (for example, Tenebrionidae). Chemical defense may serve purposes other than just protection from vertebrates, such as protection from a wide range of microbes, and repellents. Some species release chemicals in the form of a spray with surprising accuracy, such as ground beetles (Carabidae), may spray chemicals from their abdomen to repel predators. Some species take advantage of the plants from which they feed, and sequester the chemicals from the plant that would protect it and incorporate into their own defense. African carabid beetles (for example, Anthia and Thermophilum) employ the same chemicals used by ants, while bombardier beetles have a their own unique separate gland, spraying potential predators from far distances.[46]
Large ground beetles and longhorn beetles may defend themselves using strong mandibles, spines or horns to forcibly persuade a predator to seek out easier prey.[46] Many species such as the rhinoceros beetle have large protrusions from their thorax and head, which can be used to defend themselves from predators. Many species of weevil that feed out in the open on leaves of plants react to attack by employing a "drop-off reflex". Some combine it with thanatosis, in which they close up their appendages and "play dead".[47]
Parasitism
Over 1000 species of beetles are known to be either parasitic, predatory, or commensals in the nests of ants.[48]
A few species of beetles are actually ectoparasitic on mammals. One such species, Platypsyllus castoris, parasitises beavers (Castor spp.). This beetle lives as a parasite both as a larva and as an adult, feeding on epidermal tissue and possibly on skin secretions and wound exudates. They are strikingly flattened dorsoventrally, no doubt as an adaptation for slipping between the beavers' hairs. They also are wingless and eyeless, as are many other ectoparasites.[49]
Other parasitic beetles include those that are kleptoparasites of other invertebrates, such as the small hive beetle (Aethina tumida) that infests honey bee hives. The larvae tunnel through comb towards stored honey or pollen, damaging or destroying cappings and comb in the process. Larvae defecate in honey and the honey becomes discolored from the feces, which causes fermentation and a frothiness in the honey; the honey develops a characteristic odor of decaying oranges. Damage and fermentation cause honey to run out of combs, destroying large amounts of it both in hives and sometimes also in honey extracting rooms. Heavy infestations cause bees to abscond; though the beetle is only a minor pest in Africa, beekeepers in other regions have reported the rapid collapse of even strong colonies.[50]
Pollination
Beetle-pollinated flowers are usually large, greenish or off-white in color, and heavily scented. Scents may be spicy, fruity, or similar to decaying organic material. Most beetle-pollinated flowers are flattened or dish-shaped, with pollen easily accessible, although they may include traps to keep the beetle longer. The plants' ovaries are usually well protected from the biting mouthparts of their pollinators.[35] Beetles may be particularly important in some parts of the world such as semiarid areas of southern Africa and southern California[51] and the montane grasslands of KwaZulu-Natal in South Africa.[52]
Mutualism
Amongst most orders of insects, mutualism is not common, but some examples occur in species of Coleoptera, such as the ambrosia beetle, the ambrosia fungus, and probably bacteria. The beetles excavate tunnels in dead trees in which they cultivate fungal gardens, their sole source of nutrition. After landing on a suitable tree, an ambrosia beetle excavates a tunnel in which it releases spores of its fungal symbiont. The fungus penetrates the plant's xylem tissue, digests it, and concentrates the nutrients on and near the surface of the beetle gallery, so the weevils and the fungus both benefit. The beetles cannot eat the wood due to toxins, and uses its relationship with fungi to help overcome its host tree defenses and to provide nutrition for their larvae.[53] Chemically mediated by a bacterially produced polyunsaturated peroxide,[54] this mutualistic relationship between the beetle and the fungus is coevolved.[53][55]
Commensalism
Pseudoscorpions are small arachnids with flat, pear-shaped bodies and pincers that resemble those of scorpions (only distant relatives), usually ranging from 2 to 8 millimetres (0.08 to 0.31 in) in length.[56] Their small size allows them to hitch rides under the elytra of giant harlequin beetles to be dispersed over wide areas while simultaneously being protected from predators. They may also find mating partners as other individuals join them on the beetle. This would be a form of parasitism if the beetle were harmed in the process, but the beetle is, presumably, unaffected by the presence of the hitchhikers.[57][58]
Eusociality
Austroplatypus incompertus is eusocial, one of the few organisms outside Hymenoptera to do so, and the only species of Coleoptera.[59][60]
Relationship to humans
As pests
About 75% of beetle species are phytophagous in both the larval and adult stages, and live in or on plants, wood, fungi, and a variety of stored products, including cereals, tobacco, and dried fruits. Because many of these plants are important for agriculture, forestry, and the household, beetles can be considered pests.[32] Some of these species cause significant damage, such as the boll weevil, which feeds on cotton buds and flowers. The boll weevil crossed the Rio Grande near Brownsville, Texas, to enter the United States from Mexico around 1892,[61] and had reached southeastern Alabama by 1915. By the mid-1920s, it had entered all cotton-growing regions in the US, traveling 40 to 160 miles (60–260 km) per year. It remains the most destructive cotton pest in North America. Mississippi State University has estimated, since the boll weevil entered the United States, it has cost cotton producers about $13 billion, and in recent times about $300 million per year.[61] Many other species also have done extensive damage to plant populations, such as the bark beetle, elm leaf beetle and Asian longhorned beetle.[62] The bark beetle, elm leaf beetle and Asian longhorned beetle, among other species, have been known to nest in elm trees. Bark beetles in particular carry Dutch elm disease as they move from infected breeding sites to feed on healthy elm trees, which in turn allows the Asian longhorned beetle to continue killing more elms.[62] The spread of Dutch elm disease by the beetle has led to the devastation of elm trees in many parts of the Northern Hemisphere, notably in Europe and North America.[63]

Situations in which a species has developed immunity to pesticides are worse, as in the case of the Colorado potato beetle, Leptinotarsa decemlineata, which is a notorious pest of potato plants. Crops are destroyed and the beetle can only be treated by employing expensive pesticides, to many of which it has begun to develop resistance. Suitable hosts can include a number of plants from the potato family (Solanaceae), such as nightshade, tomato, eggplant and capsicum, as well as potatoes. The Colorado potato beetle has developed resistance to all major insecticide classes, although not every population is resistant to every chemical.[64]
Pests do not only affect agriculture, but can also even affect houses, such as the death watch beetle. The death watch beetle, Xestobium rufovillosum (family Anobiidae), is of considerable importance as a pest of older wooden buildings in Great Britain. It attacks hardwoods such as oak and chestnut, always where some fungal decay has taken or is taking place. The actual introduction of the pest into buildings is thought to take place at the time of construction.[65]
Other pest include the coconut hispine beetle, Brontispa longissima, which feeds on young leaves and damages seedlings and mature coconut palms. On September 27, 2007, Philippines' Metro Manila and 26 provinces were quarantined due to having been infested with this pest (to save the $800-million Philippine coconut industry).[66] The mountain pine beetle normally attacks mature or weakened lodgepole pine. It can be the most destructive insect pest of mature pine forests. The current infestation in British Columbia is the largest Canada has ever seen.[67]
As beneficial resources

Beetles are not only pests, but can also be beneficial, usually by controlling the populations of pests. One of the best, and widely known, examples are the ladybugs or ladybirds (family Coccinellidae). Both the larvae and adults are found feeding on aphid colonies. Other ladybugs feed on scale insects and mealybugs. If normal food sources are scarce, they may feed on small caterpillars, young plant bugs, or honeydew and nectar.[68] Ground beetles (family Carabidae) are common predators of many different insects and other arthropods, including fly eggs, caterpillars, wireworms, and others.[69]
Dung beetles (Scarabidae) have been successfully used to reduce the populations of pestilent flies and parasitic worms that breed in cattle dung. The beetles make the dung unavailable to breeding pests by quickly rolling and burying it in the soil, with the added effect of improving soil fertility, tilth, and nutrient cycling. The Australian Dung Beetle Project (1965–1985), led by Dr. George Bornemissza of the Commonwealth Scientific and Industrial Research Organisation, introduced species of dung beetle to Australia from South Africa and Europe, and effectively reduced the bush fly (Musca vetustissima) population by 90%.
Dung beetles play a remarkable role in agriculture. By burying and consuming dung, they improve nutrient recycling and soil structure.[70] They also protect livestock, such as cattle, by removing dung, which, if left, could provide habitat for pests such as flies. Therefore, many countries have introduced the creatures for the benefit of animal husbandry. In developing countries, the beetle is especially important as an adjunct for improving standards of hygiene. The American Institute of Biological Sciences reports that dung beetles save the United States cattle industry an estimated US$380 million annually through burying above-ground livestock feces.[71]
Some beetles help in a professional setting, doing things that people cannot; those of the family Dermestidae are often used in taxidermy and preparation of scientific specimens to clean bones of remaining soft tissue. The beetle larvae are used to clean skulls because they do a thorough job of cleaning, and do not leave the tool marks that taxidermists' tools do. Another benefit is, with no traces of meat remaining and no emulsified fats in the bones, the trophy does not develop the unpleasant dead odor. Using the beetle larvae means that all cartilage is removed along with the flesh, leaving the bones spotless.[72]

As food
Insects are used as human food in 80% of the world's nations.[73] Beetles are the most widely eaten insects. About 344 species are known to be used as food, usually eaten in the larval stage.[74] The mealworm is the most commonly eaten beetle species. The larvae of the darkling beetle and the rhinoceros beetle are also commonly eaten.
In art

Many beetles have beautiful and durable elytra that have been used as material in arts, with beetlewing the best example.[75] Sometimes, they are also incorporated into ritual objects for their religious significance. Whole beetles, either as-is or encased in clear plastic, are also made into objects varying from cheap souvenirs such as key chains to expensive fine-art jewelry. In parts of Mexico, beetles of the genus Zopherus are made into living brooches by attaching costume jewelry and golden chains, which is made possible by the incredibly hard elytra and sedentary habits of the genus.[76]
In ancient cultures
| ||
| ḫpr in hieroglyphs |
|---|


Some beetles were prominent in ancient cultures,[77] the most prominent being the dung beetle in Ancient Egypt. Several species of dung beetle, especially the "sacred scarab" Scarabaeus sacer, were revered by the ancient Egyptians.[78] The hieroglyphic image of the beetle may have had existential, fictional, or ontologic significance.[79] Images of the scarab in bone, ivory, stone, Egyptian faience, and precious metals are known from the Sixth Dynasty and up to the period of Roman rule. The scarab was of prime significance in the funerary cult of ancient Egypt.[80]
The scarab was linked to Khepri, the god of the rising sun, from the supposed resemblance of the dung ball rolled by the beetle to the rolling of the sun by the god.[78] Plutarch wrote:
The race of beetles has no female, but all the males eject their sperm into a round pellet of material which they roll up by pushing it from the opposite side, just as the sun seems to turn the heavens in the direction opposite to its own course, which is from west to east.[81]
In contrast to funerary contexts, some of ancient Egypt's neighbors adopted the scarab motif for seals of varying types. The best-known of these are the Judean LMLK seals (eight of 21 designs contained scarab beetles), which were used exclusively to stamp impressions on storage jars during the reign of Hezekiah.[82]
In modern cultures
Beetles still play roles in culture. One example is in insect fighting for entertainment and gambling. This sport exploits the territorial behavior and mating competition of certain species of large beetles. In the Chiang Mai district of northern Thailand, male Xylotrupes rhinoceros beetles are caught in the wild and trained for fighting. Females are held inside a log to stimulate the fighting males with their pheromones.[83]
References
- ↑ Family-group Names in Coleoptera (Insecta) - Page 18
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Powell (2009)
- ↑ Rosenzweig, Michael L. (1995). Species Diversity in Space and Time. Cambridge University Press. ISBN 9780521499521.
- 1 2 Hunt, T.; Bergsten, J; Levkanicova, Z; Papadopoulou, A; John, O. S.; Wild, R; Hammond, P. M.; Ahrens, D; Balke, M; Caterino, M. S.; Gómez-Zurita, J; Ribera, I; Barraclough, T. G.; Bocakova, M; Bocak, L; Vogler, A. P. (2007). "A Comprehensive Phylogeny of Beetles Reveals the Evolutionary Origins of a Superradiation". Science 318 (5858): 1913–1916. Bibcode:2007Sci...318.1913H. doi:10.1126/science.1146954. PMID 18096805.
- ↑ Hammond, P. M. (1992). "Species inventory", pp. 17–39 in Global Biodiversity, Status of the Earth’s Living Resources, B. Groombridge, ed. Chapman and Hall, London, ISBN 0412472406.
- ↑ Chapman, Arthur D. (2009). Numbers of Living Species in Australia and the World (PDF) (2nd ed.). Department of the Environment, Water, Heritage and the Arts. ISBN 978-0-642-56861-8. Archived from the original (PDF) on 19 October 2013.
- ↑ Hutchinson, G. Evelyn (1959). "Homage to Santa Rosalia or why are there so many kinds of animals?". The American Naturalist 93 (870): 145–159. doi:10.1086/282070. JSTOR 2458768.
- 1 2 3 4 5 6 7 8 9 10 Benisch, Christoph (2010). "Phylogeny of the beetles". The beetle fauna of Germany. Kerbtier. Retrieved March 16, 2011.
- 1 2 3 Powell (2009), p. 186
- ↑ Labandeira, C. C.; Sepkoski, J. J. (1993). "Insect diversity in the fossil record" (PDF). Science 261 (5119): 310–315. Bibcode:1993Sci...261..310L. doi:10.1126/science.11536548. PMID 11536548.
- ↑ GRATSHEV, Vadim G.; ZHERIKHIN, Vladimir V., J. J. (October 15, 2003). "Insect diversity in the fossil record" (PDF). Acta Zoologica Cracoviensia (Fossil Insects) (261): 129–138.
- ↑ Chang, H.; Zhang, F.; Ren, D. (2008). "A new genus and two new species of fossil elaterids from the Yixian Formation of Western Liaoning, China (Coleoptera: Elateridae)" (PDF). Zootaxa (1785): 54–62.
- ↑ Orekhovo-Zuyevo, A. V. A. (1993). "Jurassic and Lower Cretaceous Buprestidae (Coleoptera) from Eurasia" (PDF). Paleontological Journal (1A): 9–34.
- ↑ "New Jewel Beetles (Coleoptera: Buprestidae) from the Cretaceous of Russia, Kazakhstan, and Mongolia" (PDF). Paleontological Journal 43: 277–281. 2009. doi:10.1134/s0031030109030058.
- ↑ Chin, K.; Gill, B. D. (1996). "Dinosaurs, dung beetles, and conifers; participants in a Cretaceous food web". Palaois 11 (11): 280–285. doi:10.2307/3515235.
- ↑ Arillo, Antonio and Ortuño, Vicente M. (2008). "Did dinosaurs have any relation with dung-beetles? (The origin of coprophagy)". Journal of Natural History 42 (19&20): 1405–1408. doi:10.1080/00222930802105130.
- ↑ Béthoux, Oliver (2009). "The earliest beetle identified". Journal of Paleontology 83 (6): 931–937. doi:10.1666/08-158.1.
- ↑ Hörnschemeyer, T.; Stapf, H. "Die Insektentaphozönose von Niedermoschel (Asselian, unt. Perm; Deutschland)". Schriften der Alfred-Wegener-Stiftung (in German) (99/8): 98.
- ↑ Kukalová, J. (1969). "On the systematic position of the supposed Permian beetles, Tshecardocoleidae, with a description of a new collection from Moravia". Sborník geologických Věd, Paleontologie 11: 139–161.
- ↑ Beckemeyer, R. J.; Engel, M. S. (2008). "A second specimen of Permocoleus (Coleoptera) from the Lower Permian Wellington Formation of Noble County, Oklahoma" (PDF). Journal of the Kansas Entomological Society 81 (1): 4–7. doi:10.2317/JKES-708.01.1.
- ↑ Shcherbakov, D. E. (2008). "On Permian and Triassic insect faunas in relation to biogeography and the Permian-Triassic crisis". Paleontological Journal 42 (1): 15–31. doi:10.1007/s11492-008-1003-1.
- ↑ Ponomarenko, A. G. (2004). "Beetles (Insecta, Coleoptera) of the Late Permian and Early Triassic" (PDF). Paleontological Journal 38 (Suppl. 2): S185–S196. Archived from the original (PDF) on 11 November 2013.
- ↑ Ponomarenko, Alexandr G. (1985). "Fossil insects from the Tithonian "Solnhofener Plattenkalke" in the Museum of Natural History, Vienna" (PDF). Annalen des Naturhistorischen Museums in Wien 87 (1): 135–144.
- ↑ Yan, E. V. (2009). "A new genus of elateriform beetles (Coleoptera, Polyphaga) from the Middle-Late Jurassic of Karatau" (PDF). Paleontological Journal 43 (1): 78–82. doi:10.1134/S0031030109010080.
- 1 2 Tan, J.-J.; Ren, D. and Liu, M. (2005). "New ommatids from the Late Jurassic of western Liaoning, China (Coleoptera: Archostemata)" (PDF). Insect Science 12 (3): 207–216. doi:10.1111/j.1005-295X.2005.00026.x.
- ↑ Ponomarenko, A. G. (1997). "New beetles of the family Cupedidae from the Mesozoic of Mongolia. Ommatini, Mesocupedini, Priacmini" (PDF). Paleontological Journal 31 (4): 389–399. Archived from the original (PDF) on 2006-09-25.
- ↑ Whiting, Michael F. (2002). "Phylogeny of the holometabolous insect orders: molecular evidence". Zoologica Scripta 31 (1): 3–15. doi:10.1046/j.0300-3256.2001.00093.x.
- 1 2 Beutel, R.; Haas, F. (2000). "Phylogenetic relationships of the suborders of Coleoptera (Insecta)". Cladistics 16: 103–141. doi:10.1111/j.1096-0031.2000.tb00350.x.
- 1 2 Kukalová-Peck, J.; Lawrence, J. F. (1993). "Evolution of the hind wing in Coleoptera". Canadian Entomologist 125 (2): 181–258. doi:10.4039/Ent125181-2.
- ↑ Maddison, David R. (September 11, 2000). "Coleoptera. Beetle". Tree of Life Web Project. tolweb.org. Retrieved March 18, 2011.
- ↑ "Genomic and Morphological Evidence Converge to Resolve the Enigma of Strepsiptera". 2012. doi:10.1016/j.cub.2012.05.018.
- 1 2 3 4 5 6 7 8 Gilliott, Cedric (August 1995). Entomology (2 ed.). Springer-Verlag New York, LLC. p. 96. ISBN 0-306-44967-6.
- ↑ Bouchard, P.; Bousquet, Y.; Davies, A.; Alonso-Zarazaga, M.; Lawrence, J.; Lyal, C.; Newton, A.; Reid, C.; Schmitt, M.; Slipiński, A.; Smith, A. (2011). "Family-Group Names in Coleoptera (Insecta)". ZooKeys 88 (88): 1–972. doi:10.3897/zookeys.88.807. PMC 3088472. PMID 21594053.
- ↑ Foottit, Robert G.; Adler, Peter Holdridge (2009). Insect biodiversity: science and society. John Wiley and Sons. ISBN 1-4051-5142-0.
- 1 2 Gullan, P.J.; Cranston, P.S. (March 22, 2010). The Insects: An Outline of Entomology (4 ed.). Oxford: Wiley, John & Sons. ISBN 1-4443-3036-5.
- ↑ Carpenter, George Herbert (1899). Insects, their structure and life.
- ↑ Ivie, Michael A. (2002). Ross H. Arnett & Michael Charles Thomas, ed. American Beetles: Polyphaga: Scarabaeoidea through Curculionoidea. American Beetles 2. CRC Press. ISBN 978-0-8493-0954-0.
- ↑ Schmidt-Nielsen, Knut (January 15, 1997). "Insect Respiration". Animal Physiology: Adaptation and Environment (5th ed.). Cambridge University Press. p. 55. ISBN 0-521-57098-0.
- 1 2 Evans & Bellamy (2000)
- ↑ Scoble, MJ (1992). The Lepidoptera: Form, function, and diversity. Oxford Univ. Press. ISBN 978-1-4020-6242-1.
- ↑ Arnett, R. H., Jr. and Thomas, M. C. (2001). "Haliplidae". American Beetles, Volume 1. CRC Press, Boca Raton, Florida. pp. 138–143. ISBN 0-8493-1925-0.
- 1 2 "Mountain Pine Beetle – Beetle Love". Parks Canada. Retrieved March 13, 2011.
- ↑ Wyatt, T. D. and Foster, W. A. (1989). "Parental care in the subsocial intertidal beetle, Bledius spectabilis, in relation to parasitism by the ichneumonid wasp, Barycnemis blediator". Behaviour 110 (1–4): 76–92. doi:10.1163/156853989X00394. JSTOR 4534785.
- ↑ Hanski, Ilkka; Yves, Cambefort (1991). Dung Beetle Ecology. Princeton University Press. pp. 626–672. ISBN 0-691-08739-3.
- ↑ Lobanov, A.L. (2002). "feeding". Beetle Biology And Ecology. Beetles (Coleoptera) and Coleopterologist. Retrieved March 13, 2011.
- 1 2 3 Evans & Bellamy (2000), p. 126
- ↑ Powell (2009), p. 199
- ↑ Meyer, John R. (March 8, 2005). "Coleoptera". Department of Entomology, NC State University. Retrieved March 13, 2011.
- ↑ Stewart B. Peck (2006). "Distribution and biology of the ectoparasitic beaver beetle Platypsyllus castoris Ritsema in North America (Coleoptera: Leiodidae: Platypsyllinae)". Insecta Mundi 20 (1–2): 85–94.
- ↑ Neumann, P. and Elzen, P. J. (2004). "The biology of the small hive beetle (Aethina tumida, Coleoptera: Nitidulidae): Gaps in our knowledge of an invasive species". Apidologie 35 (3): 229–247. doi:10.1051/apido:2004010.
- ↑ Jones, G. D. and Jones, S. D. (2001). "The uses of pollen and its implication for Entomology". Neotropical Entomology 30 (3): 314–349. doi:10.1590/S1519-566X2001000300001.
- ↑ Ollerton, J.; Johnson, S. D.; Cranmer, L. and Kellie, S. (2003). "The pollination ecology of an assemblage of grassland asclepiads in South Africa". Annals of Botany 92 (6): 807–834. doi:10.1093/aob/mcg206.
- 1 2 Malloch, D. and Blackwell, M. (1993) "Dispersal biology of ophiostomatoid fungi", p. 195–206 in: Ceratocystis and Ophiostoma: Taxonomy, Ecology and Pathology, Wingfield, M.J., K.A. Seifert, and J.F. Webber (eds.). APS, St. Paul, ISBN 978-0-89054-156-2.
- ↑ Scott, JJ; Oh, DC; Yuceer, MC; Klepzig, KD; Clardy, J; Currie, CR (3 October 2008). "Bacterial protection of beetle-fungus mutualism". Science 322 (5898): 63. Bibcode:2008Sci...322...63S. doi:10.1126/science.1160423. PMC 2761720. PMID 18832638.
- ↑ Francke-Grossmann, H. (1967). "Ectosymbiosis in wood inhabiting insects". In M. Henry. Symbiosis 2. New York: Academic Press. pp. 141–205.
- ↑ "Pseudoscorpions". Insect Advice from Extension. College of Agricultural Sciences. 2011. Retrieved March 13, 2011.
- ↑ Gail, Vines (April 18, 1992). "Hitchhiking pseudoscorpions take beetles for a ride". New Scientist (1817).
- ↑ Poole, Robert W. (2004). "Ecology – Population Ecology – Commensalism". Nearctica. Archived from the original on December 12, 2010.
- ↑ "Science: The Australian beetle that behaves like a bee". New Scientist. 1992-05-09. Retrieved 2010-10-31.
- ↑ D. S. Kent & J. A. Simpson (1992). "Eusociality in the beetle Austroplatypus incompertus (Coleoptera: Curculionidae)". Naturwissenschaften 79: 86–87. Bibcode:1992NW.....79...86K. doi:10.1007/BF01131810.
- 1 2 Mississippi State University. "History of the Boll Weevil in the United States". Economic impacts of the boll weevil. Archived from the original on May 12, 2008.
- 1 2 "Invasion of the Longhorn Beetles". Smithsonian. Retrieved 19 Dec 2014.
- ↑ "Elm Leaf Beetle". University of California. May 11, 2011. Retrieved July 17, 2011.
- ↑ A. Alyokhin, M. Baker, D. Mota-Sanchez, G. Dively & E. Grafius (2008). "Colorado potato beetle resistance to insecticides". American Journal of Potato Research 85 (6): 395–413. doi:10.1007/s12230-008-9052-0.
- ↑ Adcock, Edward (2005). "Pests – Death watch beelte". Conservation and collective care. University of Oxford. Archived from the original on July 10, 2011. Retrieved July 17, 2011.
- ↑ Remo, Amy R. (September 27, 2007). "Beetles infest coconuts in Manila, 26 provinces". Philippine Daily Inquirer.
- ↑ "The Mountain Pine Beetle in British Columbia". Natural Resources Canada. August 19, 2008. Archived from the original on April 19, 2010. Retrieved June 24, 2010.
- ↑ "'Deadly ladybird' sighted in UK". BBC News. October 5, 2004. Retrieved June 17, 2010.
- ↑ Kromp, B. (1999). "Carabid beetles in sustainable agriculture: a review on pest control efficacy, cultivation aspects and enhancement". Agriculture, Ecosystems and Environment 74 (1–3): 187–228. doi:10.1016/S0167-8809(99)00037-7.
- ↑ Brown, Jacqueline; Scholtz, Clarke H.; Janeau, Jean-Louis; Grellier, Seraphine and Podwojewski, Pascal (2010). "Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties". Applied Soil Ecology 46 (1): 9–16. doi:10.1016/j.apsoil.2010.05.010.
- ↑ John E. Losey & Mace Vaughan (2006). "The economic value of ecological services provided by insects" (PDF). BioScience 56 (4): 311–323. doi:10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2.
- ↑ Tomberlin, Jeffery K. and Sanford, Michelle R. (2012). "Forensic entomology and wildlife". In Huffman, Jane E. and Wallace, John R. Wildlife Forensics: Methods and Applications. Developments in Forensic Science 6 (2nd ed.). John Wiley & Sons. pp. 81–107. ISBN 9781119954293.
- ↑ Carrington, Damian (August 1, 2010) "Insects could be the key to meeting food needs of growing global population", The Guardian. Retrieved February 27, 2011.
- ↑ Ramos-Elorduy, Julieta; Menzel, Peter (1998). Creepy crawly cuisine: the gourmet guide to edible insects. Inner Traditions / Bear & Company. p. 5. ISBN 978-0-89281-747-4.
- ↑ Life cycle of the rounded jewel beetles, Sternocera spp. วงจรชีวิตของแมลงทับกลมใช้เวลานานถึง 2 ปี – Siam Insect Zoo-Museum. Malaeng.com (2008-10-20). Retrieved on 2013-04-04.
- ↑ Ivie, Michael A. (2002). "105. Zopheridae". In Ross H. Arnett & Michael Charles Thomas. American Beetles: Polyphaga: Scarabaeoidea through Curculionoidea. Volume 2 of American Beetles. CRC Press. pp. 457–462. ISBN 978-0-8493-0954-0.
- ↑ Cambefort, Yves. Beetles as religious symbols. insects.org
- 1 2 Zabludoff, Marc (2008). Beetles. Malaysia: Michelle Bison. pp. 14–17. ISBN 978-0-7614-2532-8.
- ↑ Dollinger, André (January 2002). "Ancient Egyptian bestiary: Insects". Retrieved July 19, 2011.
- ↑ Morales-Correa, Ben (2006). "Egyptian Symbols". All-About-Egypt. Retrieved July 19, 2011.
- ↑ "Isis and Osiris", Moralia, in volume V of the Loeb Classical Library edition, 1936, now in the public domain.
- ↑ Ussishkin, David (2004). The New Archaeological Excavations at Lachish (1973-1994). Tel Aviv: Institute of Archaeology of Tel Aviv University.
- ↑ Rennesson, Stephane; Cesard, Nicolas; Grimaud, Emmanuel (2008). "Duels en miniature: la délicate mise en scène des combats de scarabées au nord de la Thaïlande" (PDF). Insectes (in French) (INRA) 3 (151).
Bibliography
- Evans, Arthur V.; Charles Bellamy (2000). An Inordinate Fondness for Beetles. University of California Press. ISBN 978-0-520-22323-3.
- Powell, Jerry A. (2009). "Coleoptera". In Vincent H. Resh & Ring T. Cardé. Encyclopedia of Insects (2nd ed.). Academic Press. p. 1132. ISBN 978-0-12-374144-8.
Further reading
- Poul Beckmann. Living Jewels: The Natural Design of Beetles. ISBN 3-7913-2528-0.
- J. Cooter & M. V. L. Barclay, ed. (2006). A Coleopterist's Handbook. Amateur Entomological Society. ISBN 0-900054-70-0.
- Beetle Larvae of the World. Entomological Society of America. ISBN 0-643-05506-1.
- David Grimaldi, Michael S. Engel. Evolution of the Insects. ISBN 0-521-82149-5.
- K. W. Harde. A Field Guide in Color to Beetles. pp. 7–24. ISBN 0-7064-1937-5.
- R. E. White (1983). Beetles. New York, NY: Houghton Mifflin Company. ISBN 0-395-91089-7.
External links
| Wikispecies has information related to: Coleoptera |
| The Wikibook Dichotomous Key has a page on the topic of: Coleoptera |
- Coleoptera from the Tree of Life Web Project
- Beetles and coleopterologists
- (German) Käfer der Welt
- Coleoptera Atlas
- Beetles – Coleoptera
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|


