Beer measurement
When drinking beer, there are many factors to be considered. Principal among them are bitterness, the variety of flavours present in the beverage, along with their intensity, alcohol content, and colour. Standards for those characteristics allow a more objective and uniform determination to be made on the overall qualities of any beer.
Colour
"Degrees Lovibond" or "°L" scale is a measure of the colour of a substance, usually beer, whiskey, or sugar solutions. The determination of the degrees lovibond takes place by comparing the colour of the substance to a series of amber to brown glass slides, usually by a colorimeter. The scale was devised by Joseph Williams Lovibond.[1] The Standard Reference Method (SRM) and European Brewery Convention (EBC) methods have largely replaced it, with the SRM giving results approximately equal to the °L.
The Standard Reference Method or SRM[2] is a system modern brewers use to measure colour intensity, roughly darkness (but see Tristimulus Colour below), of a beer or wort. The method involves the use of a spectrophotometer or photometer to measure the attenuation of light of a particular wavelength, 430 nanometres, as it passes through a sample contained in a cuvette located in the light path of the instrument.
The EBC convention also measures beer and wort colour, as well as quantifying turbidity (also known as haze) in beer.
- Colour based on Standard Reference Method (SRM)
| SRM/Lovibond | Example | Beer color | EBC |
|---|---|---|---|
| 2 | Pale lager, Witbier, Pilsener, Berliner Weisse | 4 | |
| 3 | Maibock, Blonde Ale | 6 | |
| 4 | Weissbier | 8 | |
| 6 | American Pale Ale, India Pale Ale | 12 | |
| 8 | Weissbier, Saison | 16 | |
| 10 | English Bitter, ESB | 20 | |
| 13 | Biere de Garde, Double IPA | 26 | |
| 17 | Dark lager, Vienna lager, Marzen, Amber Ale | 33 | |
| 20 | Brown Ale, Bock, Dunkel, Dunkelweizen | 39 | |
| 24 | Irish Dry Stout, Doppelbock, Porter | 47 | |
| 29 | Stout | 57 | |
| 35 | Foreign Stout, Baltic Porter | 69 | |
| 40+ | Imperial Stout | 79 |
Strength
Beer strength is the alcohol content measured by volume expressed as a percentage, that is to say, the number of millilitres of absolute alcohol in 100 ml of beer.
The most accurate method of determining the strength of a beer would be to take a quantity of beer and distill off a spirit that contains all of the alcohol that was in the beer. The alcohol content of the spirit can then be measured using a hydrometer and tables of density of alcohol and water mixtures. A simple calculation would then yield the strength of the beer. This method is accurate, but is time, energy and beer consuming.
A second method is the ebulliometer method, which uses the difference between the boiling temperature of pure water and the boiling temperature of the liquor (beer) being tested. This method is also accurate and time consuming, but uses less energy and beer.
The most common method of estimating the strength of a beer is to measure the density of the wort before fermentation and then to measure the density once the fermentation is completed, and to use these two data points in an empirical formula which estimates the alcohol content or strength of the beer.
Density
The most common method measuring the density of a liquid is with a hydrometer; hydrometers can be calibrated with a number of scales. A common scale is that of specific gravity (SG); that is to say the density of a liquid relative to the density of pure water (at a standard temperature). Specific gravity can also be measured by a pycnometer or oscillating U-tube electronic meter. Water has a SG of 1.000, absolute alcohol has a SG of 0.789. Other density scales are discussed below.
The density of the wort depends on the sugar content in the wort, the more sugar the higher the density. The fermented beer will have some residual sugar which will raise the SG, the alcohol content will lower the SG. The difference between the SG of the wort before fermentation and the SG of the beer after fermentation gives an indication of how much sugar was converted to alcohol by the yeast. The formula below[3] is one of many which can be used to estimate the beer strength.
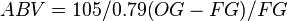
where OG is the original gravity, or the specific gravity before fermentation and FG is the final gravity of SG after fermentation.
"Original Extract" (OE) is a synonym for original gravity. The OE is often referred to as the "size" of the beer and is, in Germany, often printed on the label as Stammwürze or sometimes just as a percent. In the Czech Republic, for example, people speak of "10 degree beers", "12 degree beers" and so on.
Gravity measurements are used to determine the "size" of the beer, its alcoholic strength, and how much of the available sugar the yeast were able to consume (a given strain can be expected, under proper conditions, to ferment a wort of a particular composition to within a range of attenuation; that is, they should be able to consume a known percentage of the extract).
Other density scales
Three common scales used in fermentation are:
- balling
- brix
- plato
The oldest scale, Balling, was developed in 1843 by Bohemian scientist Karl Balling as well as Simon Ack. Adolf Brix corrected some of the calculation errors in the Balling scale and introduced the Brix table. In the early 1900s the German Fritz Plato and his collaborators made further improvements introducing the Plato scale. Essentially they are the same. The tables differ in their conversion from weight percentage to specific gravity in the fifth and sixth decimal places of the specific gravity scale.
A rough conversion between Brix, degrees Plato or degrees Balling and specific gravity can be made by dividing the number behind the decimal point in the SG (which is often referred to as gravity points) by 4. So a specific gravity of 1.048 has 48 gravity points. 48 divided by 4 is 12 degrees Plato, Balling or Brix. This conversion method is pretty accurate up to a specific gravity of 1.070 at this point the approximation begins to deviate from the actual conversion.
Winemakers as well as the sugar and juice industry typically use degrees Brix. British brewers generally use degrees Plato. American brewers use a mixture of degrees Balling, degrees Plato and specific gravity. Home wine, mead, cider, and beer makers typically use specific gravity.
In some countries, alcohol by volume is referred to as degrees Gay-Lussac (after the French chemist Joseph Louis Gay-Lussac).
XXX marks
The letter "X" is used on some beers, and was traditionally a mark of beer strength, with the more Xs the greater the strength. Some sources suggest that the origin of the mark was in the breweries of medieval monasteries.[4] Another plausible explanation is contained in a treatise entitled "The Art of Brewing" published in London in 1829. It says; "The duties on ale and beer, which were first imposed in 1643... at a certain period, in distinguishing between small beer and strong, all ale or beer, sold at or above ten shillings per barrel, was reckoned to be strong and was, therefore, subjected to a higher duty. The cask which contained this strong beer was then first marked with an X signifying ten; and hence the present denominations of XX (double X) and XXX (triple X) on the casks and accounts of the strong-ale brewers".[5]
In mid-19th century England, the use of "X" and other letters had evolved into a standardised grading system for the strength of beer.[6] Today, it is used as a trade mark by a number of brewers in the United Kingdom, the Commonwealth and the United States.
Bitterness
Bitterness scales attempt to rate the perceived relative bitterness of beer. The bitterness of beer is provided by compounds such as humulones, or alpha acids from hops used during brewing. During the brewing process, humulone undergoes isomerization to form both cis- and trans- isohumulone which are responsible for the bitter taste of the beer.[7] Likewise, hops contain lupulones, or beta acids.[7] These beta acids are not considered in the initial bittering of the wort as much as their alpha acid counterparts since they do not isomerize through boiling, and therefore do not dissolve in the wort.[8] However, beta acids can undergo oxidation and slowly contribute to the bitterness of the beer. This bitterness is harsher than the bitterness of the alpha acids and this flavor can be undesirable. The oxidation occurs over time through fermentation, storage, and aging. At the same time, isomerized alpha acids undergo degradation and reduce the bitterness of the beer.[9]
Since the quantities of alpha and beta acids range among hops, the variety of hop should be considered when targeting a specific amount of bitterness in the beer. To maximize bitterness, hops with large alpha acid concentrations should be used.[7] Such varieties include Chinook, Galena, Horizon, Tomahawk, and Warrior hops, and these contain alpha acid concentrations up to 16% by mass. Since the bitterness is not influenced by beta acids, beta acids are not considered when selecting the variety of hop. Also, the amount of time that the hops are boiled impacts the bitterness of the beer. Since heat is needed to isomerize alpha acids, applying heat for longer amounts of time increases the conversion to the isomerized form.
The International Bittering Units scale, or simply IBU scale, is the quantitative value designated to the measurement of bitterness of the beer. This scale is not measured on the perceived bitterness of the beer, but rather the amount of iso-alpha acids.[10] There are several methods to measure IBU. The most common and widely used way is through spectrophotometry.[11] In this process, hops are boiled in wort to promote isomerization. Since the iso-alpha acids are slightly hydrophobic, a reduction of the pH by adding acid increases the hydrophobicity of the iso-alpha acids. At this point, an organic solution is added and the iso-alpha acids shift to the organic layer out of the aqueous wort. This new solution is then placed in a spectrophotometer and the absorbance is read at 275 nm. At this wavelength, the iso-alpha acids have their highest absorbance which allows for the calculation of the concentration of these bittering molecules. This technique was adopted at the same time as another method based on measuring the concentration (in milligrams per litre; parts per million w/v) of IAA isomerized α acids in a beer, causing some confusion among small-scale brewers.[12] The American Society of Brewing Chemists, in the introduction to its methods on measuring bitterness, points out some differences between the results of the two methods:
While the results of the IAA methods are practically identical to those obtained by the [I]BU method for beer brewed with fresh hops, the IAAs of beer brewed with old or poorly stored hops, and with certain special hop extracts, can be significantly lower than the [I]BU figure.[13]
Additionally, HPLC, mass spectrometry, and fluorescence spectroscopy can be employed to measure the amount of iso-alpha acids in a beer.[14][15][16]
The European Bitterness Units scale, often abbreviated as EBU, is a bitterness scale[17] in which lower values are generally "less bitter" and higher values "more bitter". The scale and method are defined by the European Brewery Convention, and the numerical value should be the same as of the International Bittering Units scale (IBU), defined in co-operation with the American Society of Brewing Chemists.[18] However, the exact process of determining EBU and IBU values differs slightly, which may in theory result with slightly smaller values for EBU than IBU.[19]
IBU isn't determined by the perceived bitterness of the taste of the beer. For example, the bittering effect of hops is less noticeable in beers with roasted malts or strong flavours, so a higher proportion of hops would be required in strong flavoured beers to achieve the same perceived bitterness in moderately flavoured beers. For example, an imperial stout may have an IBU of 50, but will taste less bitter than a pale lager with an IBU of 30, because the pale lager has a lower flavour intensity. After around 100 IBU, hop utilization is so poor that the number ceases to be meaningful in regard to taste, although continued hop additions will increase bitterness. Light lagers without much bitterness will generally have 8-20 IBU, while an India pale ale may have 60-100 IBU or more.[20]
Automated combined systems
For high-through-put applications (as in quality control labs of big breweries for example), automated systems are available. Simple systems work with adjustment data blocks for each kind of beer, high-end systems are matrix-independent and give correct results for e.g. alcohol strength, extract content, pH, colour, turbidity, CO2 and O2 without any product-specific calibration.
Latest innovations are packaged beverage analyzers, that measure directly out of the package (glass bottle, PET bottle or can) and give several parameters in one measuring cycle without any sample preparation (no degassing, no filtering, no temperature conditioning).[21]
Oxidative degradation measurement
Oxidative deterioration of beer can be measured by the way of chemiluminescence[22] or by electron spin resonance.[23] Automated systems exist to determine the lag time of beer related to the antioxidant capacity to resist oxidative spoilage of flavours.[24]
Software
Software tools are available to brewers to formulate and adapt recipes with a view to accurately measure the various values in brewing. Data can be exchanged in formats such as BeerXML to allow for accurate replication of recipes at remote sites or the adaptation of recipes to account for variations in locally available water, mash ingredients, hops etc.
See also
- Beer style, information on the styles of beer
Notes and references
- ↑ Article at BrewWiki.com
- ↑ "Beer 10-A Spectrophotometric Color Method", ASBC Methods of Analysis
- ↑ "Calculate Percent Alcohol in Beer". brewmorebeer.com. Retrieved 2015-08-23.
- ↑ Bamforth 2008, p. 34-.
- ↑ Booth 1829, p. 2–.
- ↑ http://www.europeanbeerguide.net/beer_strengths_1860_1900.pdf
- 1 2 3 De Keukeleire, Denis (2000). "Fundamentals of Beer and Chemistry". Quimica Nova 23 (1): 108. doi:10.1590/S0100-40422000000100019.
- ↑ Daniels, Ray. "Alpha & Beta Acids". The Hopyard.
- ↑ "Hops: Anatomy and Chemistry 101".
- ↑ Peacock, Val. "International Bitterness Unit". Sizes.
- ↑ Blankemeier, Rick. "The Spectrophotometer and Beer: A Love Story". Hatch.
- ↑ "What Is an IBU…Really?". Basic Brewing Radio. Season 4. Episode 12. 2008-03-20.
- ↑ "Beer Bitterness (Beer-23)". Methods of Analysis (American Society of Brewing Chemists): Beer – 23:1–4. 1996.
- ↑ Jaskula, Barbara; Goiris, Koen; De Rouck, Gert; Aerts, Guido; De Cooman, Luc (2007). "Enhanced Quantitative Extraction and HPLC Determination of Hop and Beer Bitter Acids". Journal of the Institute of Brewing 113 (4): 381. doi:10.1002/j.2050-0416.2007.tb00765.x.
- ↑ "HPLC/MS/MS Analysis of Bitter Acids in Hops and Beer" (PDF). Applied Biosystems.
- ↑ Christensen, Jakob; Ladefoged, Anne; Norgaad, Lars (2005). "Rapid Detection of Bitterness in Beer Using Fluoescence Spectroscopy and Chemometrics". Journal of the Institute of Brewing 111 (1): 3. doi:10.1002/j.2050-0416.2005.tb00642.x/e.
- ↑ Lehigh Valley Homebrewers (2007). "Beer and Brewing Glossary". Retrieved 2009-08-05.
IBUs (International Bittering Units) - The accepted worldwide standard for measuring bitterness in beer, also known as EBU, based on the estimated alpha acid percentage of the hops used and the length of time they are boiled.
- ↑ European Brewery Convention. "The Analysis Committee". Retrieved 2009-08-05.
The EBC Analysis Committee also works closely together with the 'American Society of Brewing Chemists' (ASBC) to establish so-called 'International methods' with world-wide recognition of applicability. A partnership declaration between EBC and ASBC has been signed. The integration of the IOB methods of analysis and EBC methods is nearing completion.
- ↑ ajdelange (2009-06-11). "Difference between IBU and EBU". Retrieved 2009-08-05.
Because the absorption decreases pretty quickly with time at the completion of extraction the EBC reported value will, in general, be a little smaller than ASBC reported value unless the beer requires centrifugation. For all practical considerations the two systems should give the same results.
- ↑ Crouch 2006, p. 263–.
- ↑ "Anton Paar". www.anton-paar.com.
- ↑ Kaneda et al. 1990.
- ↑ Kaneda et al. 1988.
- ↑ e-scan-beer-method
- Rabin, Dan; Forget, Carl (1998). The Dictionary of Beer and Brewing. Taylor & Francis. ISBN 978-1-57958-078-0.
- Crouch, Andy (2006). The Good Beer Guide to New England. UPNE. ISBN 978-1-58465-469-8.
- Booth, David (1829). The Art of Brewing. Baldwin and Cradock.
- Bamforth, Charles W. (2008). Beer: Health and Nutrition. John Wiley & Sons. ISBN 978-1-4051-4797-2.
- Kaneda, Hirotaka; Kano, Yukinobu; Kamimura, Minoru; Osawa, Toshihiko; Kawakishi, Shunro (1990). "Detection of Chemiluminescence Produced during Beer Oxidation". Journal of Food Science 55 (3): 881–882. doi:10.1111/j.1365-2621.1990.tb05260.x. ISSN 0022-1147.
- Kaneda, Hirotaka; Kano, Yukinobu; Osawa, Toshihiko; Ramarathnam, Narasimhan; Kawakishi, Shunro; Kamada, Kozo (1988). "Detection of Free Radicals in Beer Oxidation". Journal of Food Science 53 (3): 885–888. doi:10.1111/j.1365-2621.1988.tb08978.x. ISSN 0022-1147.
External links
| ||||||