Balamuthia mandrillaris
| Balamuthia mandrillaris | |
|---|---|
 | |
| Balamuthia in cyst form | |
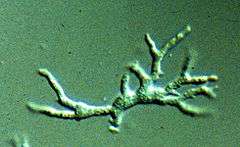 | |
| Balamuthia mandrillaris in active form | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Amoebozoa |
| Class: | Lobosea |
| Order: | Centramoebida |
| Family: | Balamuthiidae |
| Genus: | Balamuthia Visvesvara et al., 1993 |
| Species: | B. mandrillaris |
| Binomial name | |
| Balamuthia mandrillaris Visvesvara et al., 1993 | |
Balamuthia mandrillaris is a free-living amoeba that is known to cause the deadly neurological condition known as granulomatous amoebic encephalitis (GAE).[1] B. mandrillaris is found in the environment and was first discovered in 1986 in the brain of a baboon that died in the San Diego Wild Animal Park. B. mandrillaris can infect the body through skin wounds or by inhaling the dust containing Balamuthia.[2] Balamuthia has not been definitively isolated in nature, but it is believed to be distributed throughout the temperate regions of the world. This is supported somewhat by the presence of antibodies to Balamuthia present in healthy individuals. The Balamuthia genus is named in honor of the late parasitologist William Balamuth (1914–1981) for his contributions to the studies of parasitic and free-living amoebas.
Morphology
Balamuthia mandrillaris is a free-living, heterotrophic amoeba, consisting of a standard complement of organelles surrounded by a three-layered cell wall, and with an abnormally large cell nucleus. On average, a Balamuthia trophozoite is approximately 30 to 120 micrometres in diameter. The cysts fall approximately in this range as well.[3]
Life cycle
Balamuthia's life cycle, like the Acanthamoeba, consists of a cystic stage and a trophozoite stage, both of which are infectious, and both of which can be identified as inclusions in the brain tissue on microscopic examination of brain biopsies performed on infected individuals. The trophozoite is pleomorphic and uninucleated, but binucleate forms are occasionally seen. Cysts are also uninucleated possessing three walls: an outer thin irregular ectocyst, an inner thick endocyst, and a middle amorphous fibrillar mesocyst.[4] Can survive lack of food, and extremes of temperature: from freezing 0 °C (32 °F) to 70 °C (158 °F), allowing for survival outside of hosts. Their surrounding membranes are nigh impenetrable, basically resistant to all known drugs. Fatatlity rate for hosts is stated as 87 - 95%. [5] @ 29 min mark of episode
Pathology
Balamuthia mandrillaris is larger than human leukocytes therefore making phagocytosis impossible, the immune system attempts to contain them at the portal of entry by mounting type IV hypersensitivity reaction.[6] They may enter the body through the lower respiratory tract or through open wounds. Upon introduction, the amoeba may form a skin lesion, or may migrate to the brain, causing a condition known as granulomatous amoebic encephalitis,[7] (GAE), which is usually fatal. This granulomatous feature is mostly seen in immunocompetent patients, immunocompromised individuals exhibit a "peri-vascular cuffing".[8] The symptoms of infection by Balamuthia are unclear, as only a few definitive cases of Balamuthia infection have been described thus far. Balamuthia-induced GAE can cause focal paralysis, seizures, and brainstem symptoms such as facial paralysis, difficulty swallowing, and double vision.
Balamuthia is also known to cause a variety of non-neurological symptoms, and often causes skin lesions, which can progress to GAE. Many patients experiencing this particular syndrome report a skin lesion (often similar to those caused by MRSA), which does not respond well to topical antibiotics. The lesion is usually localised and very slow to heal, or fails to heal altogether. In some presentations, the infection may be mistaken for certain forms of skin cancer or leishmaniasis. Balamuthia lesions on the face often cause swelling.
Culturing and identification
Balamuthia is most easily identifiable in a brain biopsy performed on an individual suffering from GAE. The amoeba cannot be cultured on an agar plate coated with bacteria because unlike Naegleria, Balamuthia mandrillaris does not feed on bacteria. Instead Balamuthia must be cultured on primate liver or human brain microvascular endothelial cells (the cells that constitute the blood–brain barrier).[9]
Treatment
Balamuthia infection has had successful treatments. In two cases, both were treated with a cocktail of antibiotics and antifungal drugs, although it is unclear if any or all of these medications played a part in treatment. Both victims suffered permanent neurological deficits as a result of their infection. Another two cases were presented and both of these individuals received successful treatments due to discovering the infection early. Two individuals, a five-year-old girl and a 64-year-old man, developed GAE. After diagnosis, they were given powerful antimicrobial therapy. Both patients recovered.[10]
Organ transplantation
According to a MMWR report published in September 2010, two confirmed cases of Balamuthia transmission occurred through organ transplantation in December 2009 in Mississippi. Two kidney recipients, a 31-year-old woman and a 27-year-old man, suffered from post transplant encephalitis due to balamuthia. The woman died in February 2010 and the man survived with partial paralysis of right arm. The CDC was notified by a physician on December 14, 2009 about possible transplant transmission in these two patients. Histopathologic testing of donor and recipient tissues confirmed the transmission. Two other patients who received heart and liver transplants from the same donor but in different hospitals were placed on preemptive therapy and remain unaffected. A second cluster of transplant transmitted Balamuthia in Arizona was reported in the same weekly report. There were four recipients: two from Arizona (liver and kidney-pancreas), one from California (kidney) and another from Utah (heart). Recipients from Arizona—a 56-year-old male and a 24-year-old male—both succumbed to encephalitis within a span of 40 days from transplantation. The other two were placed on preemptive therapy after the first two were reported and remain unaffected.
References
- ↑ http://www.dailymail.co.uk/news/article-3026932/Mother-reveals-horror-losing-newlywed-young-daughter-BRAIN-EATING-AMOEBA-contracted-family-vacation.html
- ↑ "Balamuthia mandrillaris ameba infection". Centers for Disease Control and Prevention. Retrieved 14 June 2014.
- ↑ Dunnebacke TH, Schuster FL, Yagi S, Booton GC (September 2004). "Balamuthia mandrillaris from soil samples". Microbiology (Reading, Engl.) 150 (Pt 9): 2837–42. doi:10.1099/mic.0.27218-0. PMID 15347743.
- ↑ http://studfier.com/docs/books/Biology/Microbio/Guerrant%20TIDs/Guerrant%20TIDs%20095.pdf
- ↑ https://www.youtube.com/watch?v=kgktm9yPwb0
- ↑ Abdul Mannan Baig. Pathogenesis of amoebic encephalitis: Are the amoebas being credited to an 'inside job' done by the host immune response? Acta Trop. 2015 Apr
- ↑ Di Gregorio, C; Rivasi F; Mongiardo N; De Rienzo B; Wallace S; Visvesvara GS (December 1992). "Acanthamoeba meningoencephalitis in a patient with acquired immunodeficiency syndrome". Archives of Pathology & Laboratory Medicine 116 (12): 1363–5. PMID 1456885.
- ↑ Abdul Mannan Baig. Granulomatous amoebic encephalitis: ghost response of an immunocompromised host? J Med Microbiol. 2014 Dec;63(Pt 12):1763-6. doi:
- ↑ Martínez AJ, Visvesvara GS (March 2001). "Balamuthia mandrillaris infection". J. Med. Microbiol. 50 (3): 205–7. PMID 11232763.
- ↑ Deetz, T. R.; Sawyer, M. H.; Billman, G.; Schuster, F. L.; Visvesvara, G. S. (15 November 2003). "Successful Treatment of Balamuthia Amoebic Encephalitis: Presentation of 2 Cases". Clinical Infectious Diseases. pp. 1304–1312. doi:10.1086/379020. Retrieved 14 June 2014.
External links
- http://www.cdc.gov/balamuthia/index.html for images: Cyst of B. mandrillaris and Trophozoite of B. mandrillaris in culture. Credit: DPDx