B cell

B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype.[1] They function in the humoral immunity component of the adaptive immune system by secreting antibodies.[1] Additionally, B cells present antigen (they are also classified as professional antigen-presenting cells (APCs)) and secrete cytokines.[1]
In mammals, B cells mature in the bone marrow, which is at the core of most bones.[2] In birds, B cells mature in the bursa of Fabricius, a lymphoid organ. (the "B" from B cells comes from the name of this organ), where it was first discovered by Chang and Glick,[2] and not from bone marrow as commonly believed.
B cells, unlike the other two classes of lymphocytes, T cells and natural killer cells, express B cell receptors (BCRs) on their cell membrane.[1] BCRs allow the B cell to bind a specific antigen, against which it will initiate an antibody response.[1]
Development
B cells develop from hematopoietic stem cells (HSCs) that originate from bone marrow.[3] HSCs first differentiate into multipotent progenitor (MPP) cells, then common lymphoid progenitor (CLP) cells.[3] From here, their development into B cells occurs in several stages (shown in image to the right), each marked by various gene expression patterns and immunoglobulin H chain and L chain gene loci arrangements, the latter due to B cells undergoing V(D)J recombination as they develop.[4]
B cells undergo two types of selection while developing in the bone marrow to ensure proper development. Positive selection occurs through antigen-independent signaling involving both the pre-BCR and the BCR.[5][6] If these receptors do not bind to their ligand, B cells do not receive the proper signals and cease to develop.[5][6] Negative selection occurs through the binding of self-antigen with the BCR; If the BCR can bind strongly to self-antigen, then the B cell undergoes one of four fates: clonal deletion, receptor editing, anergy, or ignorance (B cell ignores signal and continues development).[6] This negative selection process leads to a state of central tolerance, in which the mature B cells don't bind with self antigens present in the bone marrow.[4]
To complete development, immature B cells migrate from the bone marrow to the spleen as well as pass through two transitional stages: T1 and T2.[7] Throughout their migration to the spleen and after spleen entry, they are considered T1 B cells.[8] Within the spleen, T1 B cells transition to T2 B cells.[8] T2 B cells differentiate into either follicular (FO) B cells or marginal zone (MZ) B cells depending on signals received through the BCR and other receptors.[9] Once differentiated, they are now considered mature B cells, or naive B cells.[8]
While immature and during the T1 phase, B cells express BCRCR of class IgH, but BCR expression changes to the classes IgM and IgD after transition into the T2 phase and while mature up to activation.[8]
Activation
B cell activation occurs in the secondary lymphoid organs (SLOs), such as the spleen and lymph nodes.[1] After B cells mature in the bone marrow, they migrate through the blood to SLOs, which receive a constant supply of antigen through circulating lymph.[10] At the SLO, B cell activation begins when the B cell binds to an antigen via its BCR.[11] The antigen can either be free-floating or presented by APCs such as macrophages or dendritic cells (DCs),[11] and include proteins, glycoproteins, polysaccharides, whole virus particles, and whole bacterial cells.[1] Of the three B cell subsets, FO B cells preferentially undergo T cell-dependent activation while MZ B cells and B1 B cells preferentially undergo T cell-independent activation.[12]
B cell activation is enhanced through the activity of CD21, a surface receptor in complex with surface proteins CD19 and CD81 (all three are collectively known as the B cell coreceptor complex).[13] When a BCR binds an antigen tagged with a fragment of the C3 complement protein, CD21 binds the C3 fragment, co-ligates with the bound BCR, and signals are transduced through CD19 and CD81 to lower the activation threshold of the cell.[14]
T cell-dependent activation
Antigens that activate B cells with the help of T-cell are known as T cell-dependent (TD) antigens and include foreign proteins.[1] They are named as such because they are unable to induce a humoral response in organisms that lack T cells.[1] B cell response to these antigens takes multiple days, though antibodies generated have a higher affinity and are more functionally versatile than those generated from T cell-independent activation.[1]
Once a BCR binds a TD antigen, the antigen is taken up into the B cell through receptor-mediated endocytosis, degraded, and presented to T cells as peptide pieces in complex with MHC-II molecules on the cell membrane.[15] T helper (TH) cells, typically follicular T helper (TFH) cells, that were activated with the same antigen recognize and bind these MHC-II-peptide complexes through their T cell receptor (TCR).[16] Following TCR-MHC-II-peptide binding, T cells express the surface protein CD40L as well as cytokines such as IL-4 and IL-21.[16] CD40L serves as a necessary co-stimulatory factor for B cell activation by binding the B cell surface receptor CD40, which promotes B cell proliferation, immunoglobulin class switching, and somatic hypermutation as well as sustains T cell growth and differentiation.[1] T cell-derived cytokines bound by B cell cytokine receptors also promote B cell proliferation, immunoglobulin class switching, and somatic hypermutation as well as guide differentiation.[16] After B cells receive these signals, they are considered activated.[16]
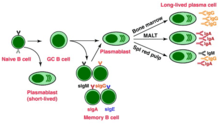
Now activated, B cells participate in a two-step differentiation process that yields both short-lived plasmablasts for immediate protection and long-lived plasma cells and memory B cells for persistent protection.[12] The first step, known as the extrafollicular response, occurs outside of lymphoid follicles but still in the SLO.[12] During this step activated B cells proliferate, may undergo immunoglobulin class switching, and differentiate into plasmablasts that produce early, weak antibodies mostly of class IgM.[17] The second step consists of activated B cells entering a lymphoid follicle and forming a germinal center (GC), which is a specialized microenvironment where B cells undergo extensive proliferation, immunoglobulin class switching, and affinity maturation directed by somatic hypermutation.[18] These processes are facilitated by TFH cells within the GC and generate both high-affinity memory B cells and long-lived plasma cells.[12] Resultant plasma cells secrete large amounts of antibody and either stay within the SLO or, more preferentially, migrate to bone marrow.[18]
T cell-independent activation
Antigens that activate B cells without T cell help are known as T cell-independent (TI) antigens[1] and include foreign polysaccharides and unmethylated CpG DNA.[12] They are named as such because they are able to induce a humoral response in organisms that lack T cells.[1] B cell response to these antigens is rapid, though antibodies generated tend to have lower affinity and are less functionally versatile than those generated from T cell-dependent activation.[1]
As with TD antigens, B cells activated by TI antigens need additional signals to complete activation, but instead of receiving them from T cells, they are provided either by recognition and binding of a common microbial constituent to toll-like receptors (TLRs) or by extensive crosslinking of BCRs to repeated epitopes on a bacterial cell.[1] B cells activated by TI antigens go on to proliferate outside of lymphoid follicles but still in SLOs (GCs do not form), possibly undergo immunoglobulin class switching, and differentiate into short-lived plasmablasts that produce early, weak antibodies mostly of class IgM, but also some populations of long-lived plasma cells.[19]
Memory B cell activation
Memory B cell activation begins with the detection and binding of their target antigen, which is shared by their parent B cell.[20] Some memory B cells can be activated without T cell help, such as certain virus-specific memory B cells, but others need T cell help.[21] Upon antigen binding, the memory B cell takes up the antigen through receptor-mediated endocytosis, degrades it, and presents it to T cells as peptide pieces in complex with MHC-II molecules on the cell membrane.[20] Memory T helper (TH) cells, typically memory follicular T helper (TFH) cells, that were derived from T cells activated with the same antigen recognize and bind these MHC-II-peptide complexes through their TCR.[20] Following TCR-MHC-II-peptide binding and the relay of other signals from the memory TFH cell, the memory B cell is activated and differentiates either into plasmablasts and plasma cells via an extrafollicular response or enter a germinal center reaction where they generate plasma cells and more memory B cells.[20][21] It is unclear whether the memory B cells undergo further affinity maturation within these secondary GCs.[20]
B cell types
- Plasmablast - A short-lived, proliferating antibody-secreting cell arising from B cell differentiation.[1] Plasmablasts are generated early in an infection and their antibodies tend to have a weaker affinity towards their target antigen compared to plasma cell.[12] Plasmablasts can result from T cell-independent activation of B cells or the extrafollicular response from T cell-dependent activation of B cells.[1]
- Plasma cell - A long-lived, non-proliferating antibody-secreting cell arising from B cell differentiation.[1] There is evidence that B cells first differentiate into a plasmablast-like cell, then differentiate into a plasma cell.[12] Plasma cells are generated later in an infection and, compared to plasmablasts, have antibodies with a higher affinity towards their target antigen due to affinity maturation in the germinal center (GC) and produce more antibodies.[12] Plasma cells typically result from the germinal center reaction from T cell-dependent activation of B cells, however they can also result from T cell-independent activation of B cells.[19]
- Memory B cell - Dormant B cell arising from B cell differentiation.[1] Their function is to circulate through the body and initiate a stronger, more rapid antibody response (known as the secondary antibody response) if they detect the antigen that had activated their parent B cell (memory B cells and their parent B cells share the same BCR, thus they detect the same antigen).[21] Memory B cells can be generated from T cell-dependent activation through both the extrafollicular response and the germinal center reaction as well as from T cell-independent activation of B1 cells.[21]
- Follicular (FO) B Cell (also known as a B-2 cell) - Most common type of B cell and, when not circulating through the blood, is found mainly in the lymphoid follicles of secondary lymphoid organs (SLOs).[12] They are responsible for generating the majority of high-affinity antibodies during an infection.[1]
- Marginal zone (MZ) B cell - Found mainly in the marginal zone of the spleen and serves as a first line of defense against blood-borne pathogens, as the marginal zone receives large amounts of blood from the general circulation.[22] They can undergo both T cell-independent and T cell-dependent activation, but preferentially undergo T cell-independent activation.[12]
- B-1 cell - Arises from a developmental pathway different from FO B cells and MZ B cells.[23] In mice, they predominantly populate the peritoneal cavity and pleural cavity, generate natural antibodies (antibodies produced without infection), defend against mucosal pathogens, and primarily exhibit T cell-independent activation.[23] A true homologue of mouse B-1 cells has not been discovered in humans, though various cell populations similar to B-1 cells have been described.[23]
- B-2 cell - FO B cells and MZ B cells.[23]
- Regulatory B (Breg) cell - An immunosuppressive B cell type that stops the expansion of pathogenic, pro-inflammatory lymphocytes through the secretion of IL-10, IL-35, and TGF-β.[24] Also, it promotes the generation of regulatory T (Treg) cells by directly interacting with T cells to skew their differentiation towards Tregs.[24] No common Breg cell identity has been described and many Breg cell subsets sharing regulatory functions have been found in both mice and humans.[24] It is currently unknown if Breg cell subsets are developmentally linked and how exactly differentiation into a Breg cell occurs.[24] There is evidence showing that nearly all B cell types can differentiate into a Breg cell through mechanisms involving inflammatory signals and BCR recognition.[24]
B cell-related pathology
Autoimmune disease can result from abnormal B cell recognition of self-antigens followed by the production of autoantibodies.[25] Autoimmune diseases where disease activity is correlated with B cell activity include scleroderma, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes, and rheumatoid arthritis.[25]
Malignant transformation of B cells and their precursors can cause a host of cancers, including chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), hairy cell leukemia, follicular lymphoma, non-Hodgkin's lymphoma, and Hodgkin's lymphoma.[26]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Murphy, Kenneth (2012). Janeway's Immunobiology 8th Edition. New York, NY: Garland Science. ISBN 9780815342434.
- 1 2 Cooper, Max D. (2015-01-01). "The early history of B cells". Nature Reviews Immunology 15 (3): 191–7. doi:10.1038/nri3801. PMID 25656707.
- 1 2 Kondo, Motonari (2010-11-01). "Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors". Immunological Reviews 238 (1): 37–46. doi:10.1111/j.1600-065X.2010.00963.x. ISSN 1600-065X. PMC 2975965. PMID 20969583.
- 1 2 Pelanda, Roberta; Torres, Raul M. (2012-04-01). "Central B-Cell Tolerance: Where Selection Begins". Cold Spring Harbor Perspectives in Biology 4 (4): a007146. doi:10.1101/cshperspect.a007146. ISSN 1943-0264. PMC 3312675. PMID 22378602.
- 1 2 Martensson, Inga-Lill; Almqvist, Nina; Grimsholm, Ola; Bernardi, Angelina (2010). "The pre-B cell receptor checkpoint". FEBS Letters 584 (12): 2572–9. doi:10.1016/j.febslet.2010.04.057. PMID 20420836.
- 1 2 3 LeBien, Tucker W.; Tedder, Thomas F. (2008-09-01). "B lymphocytes: how they develop and function". Blood 112 (5): 1570–1580. doi:10.1182/blood-2008-02-078071. ISSN 0006-4971. PMC 2518873. PMID 18725575.
- ↑ Loder, By Florienne; Mutschler, Bettina; Ray, Robert J.; Paige, Christopher J.; Sideras, Paschalis; Torres, Raul; Lamers, Marinus C.; Carsetti, Rita (1999-07-01). "B Cell Development in the Spleen Takes Place in Discrete Steps and Is Determined by the Quality of B Cell Receptor–Derived Signals". The Journal of Experimental Medicine 190 (1): 75–90. doi:10.1084/jem.190.1.75. ISSN 0022-1007. PMID 10429672.
- 1 2 3 4 Chung, James B.; Silverman, Michael; Monroe, John G. (2003-01-06). "Transitional B cells: step by step towards immune competence". Trends in Immunology 24 (6): 342–348. doi:10.1016/S1471-4906(03)00119-4. ISSN 1471-4906.
- ↑ Cerutti, Andrea; Cols, Montserrat; Puga, Irene (2013-01-01). "Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes". Nature Reviews Immunology 13 (2): 118–32. doi:10.1038/nri3383. PMC 3652659. PMID 23348416.
- ↑ Harwood, Naomi E.; Batista, Facundo D. (2010-01-01). "Early Events in B Cell Activation". Annual Review of Immunology 28 (1): 185–210. doi:10.1146/annurev-immunol-030409-101216. PMID 20192804.
- 1 2 Yuseff, Maria-Isabel; Pierobon, Paolo; Reversat, Anne; Lennon-Duménil, Ana-Maria (2013-01-01). "How B cells capture, process and present antigens: a crucial role for cell polarity". Nature Reviews Immunology 13 (7): 475. doi:10.1038/nri3469. PMID 23797063.
- 1 2 3 4 5 6 7 8 9 10 Nutt, Stephen L.; Hodgkin, Philip D.; Tarlinton, David M.; Corcoran, Lynn M. (2015-01-01). "The generation of antibody-secreting plasma cells". Nature Reviews Immunology 15 (3): 160. doi:10.1038/nri3795. PMID 25698678.
- ↑ Asokan, Rengasamy; Banda, Nirmal K.; Szakonyi, Gerda; Chen, Xiaojiang S.; Holers, V. Michael (2013-01-01). "Human complement receptor 2 (CR2/CD21) as a receptor for DNA: Implications for its roles in the immune response and the pathogenesis of systemic lupus erythematosus (SLE)". Molecular Immunology 53 (1–2): 99–110. doi:10.1016/j.molimm.2012.07.002. PMC 3439536. PMID 22885687.
- ↑ Zabel, Mark D.; Weis, John H. (2001-03-01). "Cell-specific regulation of the CD21 gene". International Immunopharmacology. Unraveling Mechanisms and Discovering Novel Roles for Complement 1 (3): 483–493. doi:10.1016/S1567-5769(00)00046-1. PMID 11367532.
- ↑ Blum, Janice S.; Wearsch, Pamela A.; Cresswell, Peter (2013-01-01). "Pathways of Antigen Processing". Annual Review of Immunology 31 (1): 443–473. doi:10.1146/annurev-immunol-032712-095910. PMC 4026165. PMID 23298205.
- 1 2 3 4 Crotty, Shane (2015-01-01). "A brief history of T cell help to B cells". Nature Reviews Immunology 15 (3): 185–9. doi:10.1038/nri3803. PMC 4414089. PMID 25677493.
- ↑ MacLennan, Ian C. M.; Toellner, Kai-Michael; Cunningham, Adam F.; Serre, Karine; Sze, Daniel M.-Y.; Zúñiga, Elina; Cook, Matthew C.; Vinuesa, Carola G. (2003-08-01). "Extrafollicular antibody responses". Immunological Reviews 194: 8–18. doi:10.1034/j.1600-065x.2003.00058.x. ISSN 0105-2896. PMID 12846803.
- 1 2 Shlomchik, Mark J.; Weisel, Florian (2012-05-01). "Germinal center selection and the development of memory B and plasma cells". Immunological Reviews 247 (1): 52–63. doi:10.1111/j.1600-065X.2012.01124.x. ISSN 1600-065X. PMID 22500831.
- 1 2 Bortnick, Alexandra; Chernova, Irene; Quinn, William J.; Mugnier, Monica; Cancro, Michael P.; Allman, David (2012-06-01). "Long-Lived Bone Marrow Plasma Cells Are Induced Early in Response to T Cell-Independent or T Cell-Dependent Antigens". The Journal of Immunology 188 (11): 5389–5396. doi:10.4049/jimmunol.1102808. ISSN 0022-1767. PMC 4341991. PMID 22529295.
- 1 2 3 4 5 McHeyzer-Williams, Michael; Okitsu, Shinji; Wang, Nathaniel; McHeyzer-Williams, Louise (2011-01-01). "Molecular programming of B cell memory". Nature Reviews Immunology 12 (1): 24–34. doi:10.1038/nri3128. PMC 3947622. PMID 22158414.
- 1 2 3 4 Kurosaki, Tomohiro; Kometani, Kohei; Ise, Wataru (2015-01-01). "Memory B cells". Nature Reviews Immunology 15 (3): 149. doi:10.1038/nri3802. PMID 25677494.
- ↑ Pillai, Shiv; Cariappa, Annaiah; Moran, Stewart T. (2005-01-01). "Marginal Zone B Cells". Annual Review of Immunology 23 (1): 161–196. doi:10.1146/annurev.immunol.23.021704.115728. PMID 15771569.
- 1 2 3 4 Baumgarth, Nicole (2010-01-01). "The double life of a B-1 cell: self-reactivity selects for protective effector functions". Nature Reviews Immunology 11 (1): 34–46. doi:10.1038/nri2901. PMID 21151033.
- 1 2 3 4 5 Rosser, Elizabeth C.; Mauri, Claudia (2015). "Regulatory B Cells: Origin, Phenotype, and Function". Immunity 42 (4): 607–612. doi:10.1016/j.immuni.2015.04.005. ISSN 1074-7613. PMID 25902480.
- 1 2 Yanaba, Koichi; Bouaziz, Jean-David; Matsushita, Takashi; Magro, Cynthia M.; St.Clair, E. William; Tedder, Thomas F. (2008-06-01). "B-lymphocyte contributions to human autoimmune disease". Immunological Reviews 223 (1): 284–299. doi:10.1111/j.1600-065X.2008.00646.x. ISSN 1600-065X.
- ↑ III, Arthur L. Shaffer; Young, Ryan M.; Staudt, Louis M. (2012-01-01). "Pathogenesis of Human B Cell Lymphomas". Annual Review of Immunology 30 (1): 565–610. doi:10.1146/annurev-immunol-020711-075027. PMID 22224767.
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||