Iniparib
 | |
| Systematic (IUPAC) name | |
|---|---|
|
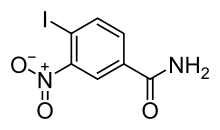
4-iodo-3-nitrobenzamide | |
| Clinical data | |
| Pregnancy category |
|
| Legal status |
|
| Identifiers | |
| CAS Number |
160003-66-7 |
| ATC code | None |
| PubChem | CID 9796068 |
| ChemSpider |
7971834 |
| UNII |
2ZWI7KHK8F |
| KEGG |
D09913 |
| ChEMBL |
CHEMBL1170047 |
| Chemical data | |
| Formula | C7H5IN2O3 |
| Molar mass | 292.03 g/mol |
| |
| |
| | |
Iniparib (previously BSI 201) (4-iodo-3-nitrobenzamide) was a drug candidate for cancer treatment. It was originally believed to act as an irreversible inhibitor of PARP1 (hence, a PARP inhibitor) and possibly other enzymes through covalent modification,[1][2] but its effects against PARP were later disproven.[3][4] It underwent clinical trials for treatment of some types of breast cancer,[5][6] but was discontinued after disappointing Phase III clinical trials.
History
Iniparib was the first putative PARP inhibitor to commence phase III clinical trials.[7] The first was for breast cancer,[8] another was for squamous cell lung cancer.[9] Preliminary results in June 2009 on triple-negative breast cancer were promising.[10] Later results showed increased median survival of triple negative breast cancer patients from 7.7 to 12.2 months.[11][12][13]
In 2009, the FDA began fast-tracking the New Drug Application of iniparib for triple-negative breast cancer. However, Phase III results disclosed in January 2011 were disappointing.[14][15]
Iniparib was also studied as a potential chemotherapeutic agent in the fight against malignant glioma, including glioblastoma. Glioma is a resilient type of primary brain tumor (not metastatic) that currently has limited effective therapies, especially for patients whose tumors are in an inoperable location of the brain, such as the interior of the brainstem.
During the 2013 American Society of Clinical Oncology conference, Sanofi disclosed that iniparib failed to help lung-cancer patients in a late-stage trial, prompting the company to end research into the once-promising compound and take a $285 million charge.[14][16]
References
- ↑ Development of PARP Inhibitors: An Unfinished Story, Jan 2010
- ↑ http://www.biparsciences.com/000011.html Archived June 23, 2010 at the Wayback Machine
- ↑ Liu, X; Shi, Y; Maag, DX; Palma, JP; Patterson, MJ; Ellis, PA; Surber, BW; Ready, DB; et al. (2012). "Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a Bona Fide PARP inhibitor". Clinical cancer research : an official journal of the American Association for Cancer Research 18 (2): 510–23. doi:10.1158/1078-0432.CCR-11-1973. PMID 22128301.
- ↑ Patel, AG; De Lorenzo, SB; Flatten, KS; Poirier, GG; Kaufmann, SH (2012). "Failure of Iniparib to Inhibit Poly(ADP-Ribose) Polymerase in Vitro". Clinical cancer research : an official journal of the American Association for Cancer Research 18 (6): 1655–62. doi:10.1158/1078-0432.CCR-11-2890. PMC 3306513. PMID 22291137.
- ↑ BSI 201
- ↑ "New breast cancer drugs block cell repair enzyme". Reuters. 2009-05-31.
- ↑ "PARP Inhibitors in Oncology. Chemosensitizers or Single-Agent Therapeutics?" (PDF). July 2009.
- ↑ "A Phase 3, Multi-Center Study of Gemcitabine/Carboplatin, With or Without BSI-201, in Patients With ER-, PR-, and Her2-Negative Metastatic Breast Cancer". 2009 to 2012, Primary completion date June 2011
- ↑ "Trial of Gemcitabine/Carboplatin With or Without BSI-201 (a PARP1 Inhibitor) in Patients With Previously Untreated Advanced Squamous Cell Lung Cancer (ECLIPSE)". 2010 to 2014, PCD 2012
- ↑ "Sanofi’s new drug BSI 201 offers to treat the toughest breast cancer". 3 June 2009.
- ↑ "SABCS: PARP Inhibitor Data Called 'Spectacular'". Dec 2009.
- ↑ "PARP Inhibitor Adds Nearly 5 Months to Breast Cancer Survival". 11 Oct 2010.
- ↑ O'Shaughnessy; Osborne, Cynthia; Pippen, John E.; Yoffe, Mark; Patt, Debra; Rocha, Christine; Koo, Ingrid Chou; Sherman, Barry M.; Bradley, Charles; et al. (2011). "Iniparib plus Chemotherapy in Metastatic Triple-Negative Breast Cancer". New England Journal of Medicine 364 (3): 205–214. doi:10.1056/NEJMoa1011418. PMID 21208101.
- 1 2 "Sanofi breast cancer drug flunks Phase III trial".
- ↑ Joyce O'Shaughnessy, Lee Schwartzberg, Michael A. Danso et al. (2014-12-01). "Phase III Study of Iniparib Plus Gemcitabine and Carboplatin Versus Gemcitabine and Carboplatin in Patients With Metastatic Triple-Negative Breast Cancer". J. Clin. Oncol. 32 (34): 3840–7. doi:10.1200/JCO.2014.55.2984. PMID 25349301. Retrieved 2015-07-24.
- ↑ "Sanofi Ends Iniparib Research".