Azastene
 | |
| Names | |
|---|---|
| Other names
Win-17625 | |
| Identifiers | |
| 13074-00-5 | |
| ChEMBL | ChEMBL2103987 |
| ChemSpider | 9900482 |
| Jmol interactive 3D | Image |
| PubChem | 11725766 |
| |
| |
| Properties | |
| C23H33NO2 | |
| Molar mass | 355.52 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Azastene is a chemical that modulates 3β-hydroxysteroid dehydrogenase activity.[1]
Synthesis
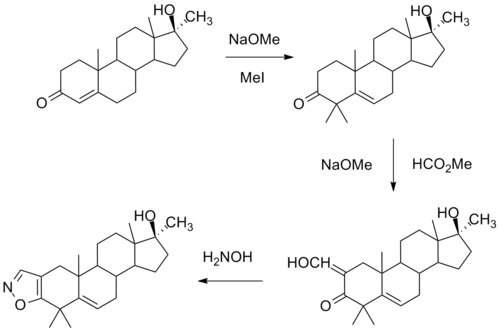
Azastene synthesis:[2]
One synthesis of this compound involves initial alkylation of methyl testosterone by means of strong base and methyl iodide to afford the 4,4-dimethyl derivative. Formylation with alkoxide and methyl formate leads to the 2-hydroxymethyl derivative. Reaction of this last with hydroxylamine leads to formation of an isoxazole ring. There is then obtained azastene.
References
- ↑ Liu, CG; Dai, MZ; Li, WK; Liu, GM; Lin, ZM; Ma, RH (1987). "Interceptive action of azastene and its effects on plasma progesterone in pregnant rats and rabbits". Zhongguo yao li xue bao = Acta pharmacologica Sinica 8 (6): 540–3. PMID 3451668.
- ↑ Gordon O. Potts, Sterling Drug Inc. U.S. Patent 3,966,926 (1976).
This article is issued from Wikipedia - version of the Saturday, July 04, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.