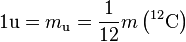Atomic mass unit
| Unified atomic mass unit (Dalton) | |
|---|---|
| Unit system |
Physical constant (Accepted for use with the SI) |
| Unit of | mass |
| Symbol | u or Da |
| Named after | John Dalton |
| Unit conversions | |
| 1 u or Da in ... | ... is equal to ... |
| kg | 1.660539040(20)×10−27 |
| MeV/c2 | 931.494095(11) |
| me | 1822.88839 |
The unified atomic mass unit (symbol: u) or dalton (symbol: Da) is the standard unit that is used for indicating mass on an atomic or molecular scale (atomic mass). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.[1] It is defined as one twelfth of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state,[2] and has a value of 1.660539040(20)×10−27 kg.[3] The CIPM has categorised it as a non-SI unit accepted for use with the SI, and whose value in SI units must be obtained experimentally.[2]
The amu without the "unified" prefix is technically an obsolete unit based on oxygen, which was replaced in 1961. However, some sources may still use the term "amu" but now define it in the same way as u (i.e., based on carbon-12). In this sense, most uses of the terms "atomic mass units" and "amu" today actually refer to unified atomic mass unit. For standardization a specific atomic nucleus (carbon-12 vs. oxygen-16) had to be chosen because the average mass of a nucleon depends on the count of the nucleons in the atomic nucleus due to mass defect. This is also why the mass of a proton (or neutron) by itself is more than (and not equal to) 1 u.
Atomic mass unit does not stand for the unit of mass in the atomic units system, which is rather me.
History
The relative atomic mass (atomic weight) scale has traditionally been a relative scale, that is without an explicit unit, with the first relative atomic mass basis suggested by John Dalton in 1803 as 1H.[4] Despite the initial mass of 1H being used as the natural unit for relative atomic mass, it was suggested by Wilhelm Ostwald that relative atomic mass would be best expressed in terms of units of 1/16 mass of oxygen. This evaluation was made prior to the discovery of the existence of elemental isotopes, which occurred in 1912.[4]
The discovery of isotopic oxygen in 1929 led to a divergence in relative atomic mass representation, with isotopically weighted oxygen (i.e., naturally occurring oxygen relative atomic mass) given a value of exactly 16 atomic mass units (amu) in chemistry, while pure 16O (oxygen-16) was given the mass value of exactly 16 amu in physics.
The divergence of these values could result in errors in computations, and was unwieldy. The chemistry amu, based on the relative atomic mass (atomic weight) of natural oxygen (including the heavy naturally-occurring isotopes 17O and 18O), was about 1.000282 as massive as the physics amu, based on pure isotopic 16O.
For these and other reasons, the reference standard for both physics and chemistry was changed to carbon-12 in 1961.[5] The choice of carbon-12 was made to minimise further divergence with prior literature.[4] The new and current unit was referred to as the "unified atomic mass unit" u.[6] and given a new symbol, "u," which replaced the now deprecated "amu" that had been connected to the old oxygen-based system. The Dalton (Da) is another name for the unified atomic mass unit.[7]
Despite this change, modern sources often still use the old term "amu" but define it as u (1/12 of the mass of a carbon-12 atom), as mentioned in the article's introduction. Therefore, in general, "amu" likely does not refer to the old oxygen standard unit, unless the source material originates from or before the 1960s.
The unified atomic mass unit u was defined as:
Terminology
The unified atomic mass unit and the dalton are different names for the same unit of measure. As with other unit names such as watt and newton, "dalton" is not capitalized in English, but its symbol Da is capitalized. With the introduction of the name "dalton", there has been a gradual change towards using that name in preference to the name "unified atomic mass unit":
- In 1993, the International Union of Pure and Applied Chemistry approved the use of the dalton with the qualification that the CGPM had not given its approval.[8]
- In 2003 the Consultative Committee for Units, part of the CIPM, recommended a preference for the usage of the "dalton" over the "unified atomic mass unit" as it "is shorter and works better with prefixes".[9]
- In 2005, the International Union of Pure and Applied Physics endorsed the use of the dalton as an alternative to the unified atomic mass unit.[10]
- In 2006, in the 8th edition of the formal definition of SI, the CIPM cataloged the dalton alongside the unified atomic mass unit as a "Non-SI unit whose values in SI units must be obtained experimentally: Units accepted for use with the SI".[2] The definition also noted that "The dalton is often combined with SI prefixes ..."
- In 2009, when the International Organization for Standardization published updated versions of ISO 80000, it gave mixed messages as to whether or not the unified atomic mass unit had been deprecated: ISO 80000-1:2009 (General), identified the dalton as having "earlier [been] called the unified atomic mass unit u",[11] but ISO 80000-10:2009 (atomic and nuclear physics) catalogued both as being alternatives for each other.[12]
- The 2010 version of the Oxford University Press style guide for authors in life sciences gave the following guidance: "Use the Système international d'unités (SI) wherever possible ... The dalton (Da) or more conveniently the kDa is a permitted non-SI unit for molecular mass or mass of a particular band in a separating gel."[13] At the same time, the author guidelines for the journal "Rapid Communications in Mass Spectrometry" stated "The dalton (Da) is a unit of mass normally used for the molecular weight ... use of the Da in place of the u has become commonplace in the mass spectrometry literature ... The "atomic mass unit", abbreviated "amu", is an archaic unit".[14]
- In 2012, in response to the proposed redefinition of the kilogram, it was proposed that the dalton be redefined as being 0.001/NA kg, thereby breaking the link with 12C. This would result in the dalton and the atomic mass unit having slightly different definitions, but the suggestion is that the older unit should be superseded by the "new" dalton.[15]
Relationship to SI
The definition of the mole, an SI base unit, was accepted by the CGPM in 1971 as:
- The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12; its symbol is "mol".
- When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
The definition of the mole also determines the value of the universal constant that relates the number of entities to amount of substance for any sample. This constant is called the Avogadro constant, symbol NA or L, and has the value 6.022140857(74)×1023 mol−1 (entities per mole).[16]
Given that the unified atomic mass unit is one twelfth the mass of one atom of carbon-12, meaning the mass of such an atom is 12 u, it follows that there are NA atoms of carbon-12 in 0.012 kg of carbon-12. This can be expressed mathematically as
- NA (12 u) = 0.012 kg/mol, or
- NA u = 0.001 kg/mol
Masses of proteins are often expressed in daltons. For example, a protein with a molecular weight of 64000 g·mol−1 has a mass of 64 kDa.[1]
Examples
- A hydrogen-1 atom has a mass of 1.0078250 u (1.0078250 Da).
- By definition, a carbon-12 atom has a mass of 12 u (12 Da).
- A molecule of acetylsalicylic acid (Aspirin) has a mass of 180.16 u (180.16 Da).
- Titin, the largest known protein, has an atomic mass of 3-3.7 megadaltons (3000000 Da).[17]
See also
Notes and references
- 1 2 Stryer, Jeremy M. Berg; John L. Tymoczko; Lubert (2007). "2". Biochemistry (6. ed., 3. print. ed.). New York: Freeman. p. 35. ISBN 978-0-7167-8724-2.
- 1 2 3 International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 126, ISBN 92-822-2213-6
- ↑ Unified Atomic mass unit. Fundamental Physical Constants from NIST
- 1 2 3 Petley, B. W., "The atomic mass unit", IEEE Trans. Instrum. Meas. 38 (2): 175–79, doi:10.1109/19.192268
- ↑ Holden, Norman E. (2004), "Atomic Weights and the International Committee—A Historical Review", Chem. Int. 26 (1): 4–7
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "unified atomic mass unit".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "dalton".
- ↑ Mills, Ian; Cvitaš, Tomislav; Homann, Klaus; Kallay, Nikola; Kuchitsu, Kozo (1993n). Quantities, Units and Symbols in Physical Chemistry International Union of Pure and Applied Chemistry; Physical Chemistry Division (PDF) (2nd ed.). International Union of Pure and Applied Chemistry and published for them by Blackwell Science Ltd. ISBN 0-632-03583-8.
- ↑ "Consultative Committee for Units (CCU); Report of the 15th meeting (17–18 April 2003) to the International Committee for Weights and Measures" (PDF). Retrieved 14 Aug 2010.
- ↑ "IU14. IUPAC Interdivisional Committee on Nomenclature and Symbols (ICTNS)". Retrieved 2010-08-14.
- ↑ International Standard ISO 80000-1:2009 – Quantities and Units – Part 1: General, International Organization for Standardization, 2009
- ↑ International Standard ISO 80000-10:2009 – Quantities and units – Part 10: Atomic and nuclear physics, International Organization for Standardization, 2009
- ↑ "Instructions to Authors". AoB Plants. Oxford journals; Oxford University Press. Retrieved 2010-08-22.
- ↑ "Author guidelines". Rapid Communications in Mass Spectrometry (Wiley-Blackwell). 2010. Retrieved 2011-05-08.
- ↑ Leonard, B P (2012). "Why the dalton should be redefined exactly in terms of the kilogram". Metrologia 49: 487–491. Bibcode:2012Metro..49..487L. doi:10.1088/0026-1394/49/4/487.
- ↑ Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). "CODATA Recommended Values of the Fundamental Physical Constants: 2006". Rev. Mod. Phys. 80 (2): 633–730. arXiv:0801.0028. Bibcode:2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633. Direct link to value.
- ↑ Opitz CA, Kulke M, Leake MC, Neagoe C, Hinssen H, Hajjar RJ, Linke WA (October 2003). "Damped elastic recoil of the titin spring in myofibrils of human myocardium". Proc. Natl. Acad. Sci. U.S.A. 100 (22): 12688–93. Bibcode:2003PNAS..10012688O. doi:10.1073/pnas.2133733100. PMC 240679. PMID 14563922.
External links
- atomic mass unit at sizes.com
| |||||||||||||||||||||||||