Atmospheric methane
Atmospheric methane is the methane present in Earth's atmosphere. Atmospheric methane concentrations are of interest because it is one of the most potent greenhouse gases in Earth's atmosphere. The 100-year global warming potential of methane is 29[1] (i.e., over a 100-year period, it traps 29 times more heat per mass unit than carbon dioxide and 32 times the effect when accounted for aerosol interactions.[2]) Global methane levels, had risen to 1800 parts per billion (ppb) by 2011, an increase by a factor of 2.5 since pre-industrial times, from 722 ppb, the highest value in at least 800,000 years.[3] Its concentration is higher in the Northern Hemisphere since most sources (both natural and human) are located on land and the Northern Hemisphere has more land mass. The concentrations vary seasonally, with a minimum in the late summer mainly due to removal by the hydroxyl radical.

Early in the Earth's history carbon dioxide and methane likely produced a greenhouse effect. The carbon dioxide would have been produced by volcanoes and the methane by early microbes. During this time, Earth's earliest life appeared.[4] These first, ancient bacteria added to the methane concentration by converting hydrogen and carbon dioxide into methane and water. Oxygen did not become a major part of the atmosphere until photosynthetic organisms evolved later in Earth's history. With no oxygen, methane stayed in the atmosphere longer and at higher concentrations than it does today.
Methane is created near the surface, and it is carried into the stratosphere by rising air in the tropics. Uncontrolled build-up of methane in Earth's atmosphere is naturally checked—although human influence can upset this natural regulation—by methane's reaction with hydroxyl radicals formed from singlet oxygen atoms and with water vapor.


Methane as a greenhouse gas

Methane in the Earth's atmosphere is a strong greenhouse gas with a global warming potential of 29 over a 100-year period. This means that a methane emission will have 29 times the impact on temperature of a carbon dioxide emission of the same mass over the following 100 years. Methane has a large effect (100 times as strong as carbon dioxide) for a brief period (having a half-life of 7 years in the atmosphere[7]), whereas carbon dioxide has a small effect for a long period (over 100 years). Because of this difference in effect and time period, the global warming potential of methane over a 20-year time period is 86.
The concentration of methane in Earth's atmosphere has increased by about 150% since 1750, and it now accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases.[8] According to NOAA the atmospheric methane concentration was measured at 1890 ppb in Ireland (Northern Hempishere) and 1760 ppb (Tasmania, Southern Hemisphere) in 2013 - arctic methane release from melting methane clathrates has played a role, but other recent emissions include peat fires in Southeast Asia. Atmospheric methane concentration has not been as high as this for over 420,000 years and correlates to an increase in average Earth temperature of 9 °C (see graph, which shows the close correlation between CH4 concentrations, CO2 concentrations, and Earth's temperature variations).
Global methane cycle
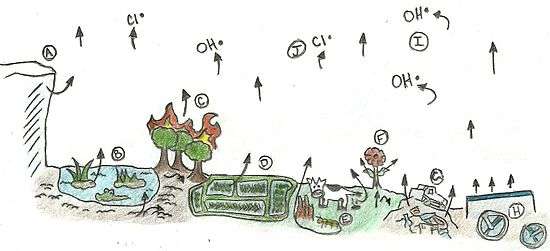
A. Permafrost, glaciers, and ice cores – A source that slowly releases methane trapped in frozen environments as global temperatures rise.
B. Wetlands – Warm temperatures and moist environments are ideal for methane production. Most of the methane makes it past methane-consuming microorganisms.
C. Forest fire – Mass burning of organic matter releases methane into the atmosphere.[9]
D. Rice paddies – The warmer and moister the rice field, the more methane is produced.
E. Animals – Microorganisms breaking down difficult to digest material in the guts of ruminant livestock and termites produce methane that is then released during defecation.
F. Plants – While methane can be consumed in soil before being released into the atmosphere, plants allow for direct travel of methane up through the roots and leaves and into the atmosphere. Plants may also be direct producers of methane.
G. Landfills – Decaying organic matter and anaerobic conditions cause landfills to be a significant source of methane.
H. Waste water treatment facilities – Anaerobic treatment of organic compounds in the water results in the production of methane.
I. Hydroxyl radical – OH in the atmosphere is the largest sink for atmospheric methane as well as one of the most significant sources of water vapor in the upper atmosphere.
J. Chlorine radical – Free chlorine in the atmosphere also reacts with methane.
Emissions accounting of methane


The balance between sources and sinks of methane is not yet fully understood. The IPCC Working Group I stated in chapter 2 of the Fourth Assessment Report that there are "large uncertainties in the current bottom-up estimates of components of the global source", and the balance between sources and sinks is not yet well known. The most important sink in the methane cycle is reaction with the hydroxyl radical, which is produced photochemically in the atmosphere. Production of this radical is not fully understood and has a large effect on atmospheric concentrations. This uncertainty is exemplified by observations that have shown between the year 2000 and 2006 increases in atmospheric concentration of methane ceased without reduction in anthropogenic sources, showing that methane accounting does not accurately predict methane observations.
Houweling et al. (1999) give the following values for methane emissions (Tg/a=teragrams per year):[11]
| Origin | CH 4 Emission | ||
|---|---|---|---|
| Mass (Tg/a) | Type (%/a) | Total (%/a) | |
| Natural Emissions | |||
| Wetlands (incl. Rice agriculture) | 225 | 83 | 37 |
| Termites | 20 | 7 | 3 |
| Ocean | 15 | 6 | 3 |
| Hydrates | 10 | 4 | 2 |
| Natural Total | 270 | 100 | 45 |
| Anthropogenic Emissions | |||
| Energy | 110 | 33 | 18 |
| Landfills | 40 | 12 | 7 |
| Ruminants (Livestock) | 115 | 35 | 19 |
| Waste treatment | 25 | 8 | 4 |
| Biomass burning | 40 | 12 | 7 |
| Anthropogenic Total | 330 | 100 | 55 |
| Sinks | |||
| Soils | -30 | -5 | -5 |
| Tropospheric OH | -510 | -88 | -85 |
| Stratospheric loss | -40 | -7 | -7 |
| Sink Total | -580 | -100 | -97 |
| Emissions + Sinks | |||
| Imbalance (trend) | +20 | ~2.78 Tg/(nmol/mol) | +7.19 (nmol/mol)/a |
Any process that results in the production of methane and its release into the atmosphere can be considered a "source." The two main processes that are responsible for methane production occur as a result of microorganisms anaerobically converting organic compounds into methane.
Methanogenesis, the scientific term for methane production, occurs primarily in anaerobic conditions because of the lack of availability of other oxidants. In these conditions, microscopic organisms called archaea use acetate and hydrogen to break down essential resources in a process called fermentation.
Acetoclastic methanogenesis- certain archaea cleave acetate produced during anaerobic fermentation to yield methane and carbon dioxide.
H3C-COOH → CH4 + CO2
Hydrogenotrophic methanogenesis- archaea oxidize hydrogen with carbon dioxide to yield methane and water.
4H2 + CO2 → CH4 + 2H2O
While acetoclastic methanogenesis and hydrogenotrophic methanogenesis are the two major source reactions for atmospheric methane, other minor biological methane source reactions also occur.
Natural sources of atmospheric methane
Most ecological emissions of methane relate directly to methanogens generating methane in warm, moist soils as well as in the digestive tracts of certain animals.
Methanogens
Methanogens are methane producing microorganisms. In order to produce energy, they use an anaerobic process called methanogenesis. This process is used in lieu of aerobic, or with oxygen, processes because methanogens are unable to metabolise in the presence of even small concentrations of oxygen. When acetate is broken down in methanogenesis, the result is the release of methane into the surrounding environment.
Wetlands
Wetlands account for approximately 20 percent of atmospheric methane through emissions from soils and plants.[12] Wetlands counteract the sinking action that normally occurs with soil because of the high water table. When the water table is low, the methane generated within the wetland soil has to come up through the soil and get past multitudes of methanotrophic bacteria. When the water table is higher, then the methane produced in the soil can more easily diffuse through the water and escape into the atmosphere.
Animals
Ruminant animals, particularly cows and sheep, contain bacteria in their gastrointestinal systems that help to break down plant material. Some of these microorganisms use the acetate from the plant material to produce methane, and because these bacteria live in the stomachs and intestines of ruminants, whenever the animal "burps" or defecates, it emits methane as well. The amount of methane emitted by one cow is equivalent to the amount of methane that 2.5 acres of methanotrophic bacteria can consume.
Termites also contain methanogenic microorganisms in their gut. However, some of these microorganisms are so unique that they live nowhere else in the world except in the third gut of termites. These microorganisms also break down biotic components to produce ethanol, as well as methane byproduct. However, unlike ruminants who lose 20 percent of the energy from the plants they eat, termites only lose 2 percent of their energy in the process.[13] Thus comparatively, termites do not have to eat as much food as ruminants to obtain the same amount of energy, and give off proportionally less methane.
Plants
Living plants (e.g. forests) have recently been identified as a potentially important source of methane, possibly being responsible for approximately 10 to 30 percent of atmospheric methane.[14] A 2006 paper calculated emissions of 62–236 Tg a−1, and "this newly identified source may have important implications".[15] [16] However the authors stress "our findings are preliminary with regard to the methane emission strength".[17]
These findings have been called into question in a 2007 paper which found "there is no evidence for substantial aerobic methane emission by terrestrial plants, maximally 0.3% of the previously published values".[18]
While the details of plant methane emissions have yet to be confirmed, plants as a significant methane source would help fill in the gaps of previous global methane budgets as well as explain large plumes of methane that have been observed over the tropics.[14][19]
In wetlands, where rate of methane production are high, plants help methane travel into the atmosphere—acting like inverted lightning rods as they direct the gas up through the soil and into the air. They are also suspected to produce methane themselves, but because the plants would have to use aerobic conditions to produce methane, the process itself is still unidentified.
Methane gas from methane clathrates
At high pressures, such as are found on the bottom of the ocean, methane forms a solid clathrate with water, known as methane hydrate. An unknown, but possibly very large quantity of methane is trapped in this form in ocean sediments. The release of large volumes of methane gas from such sediments into the atmosphere has been suggested as a possible cause for rapid global warming events in the Earth's distant past, such as the Paleocene–Eocene Thermal Maximum of 55 million years ago, and the Great Dying.
Theories suggest that should global warming cause them to heat up sufficiently, all of this methane gas could again be released into the atmosphere. Since methane gas is twenty-five times stronger (for a given weight, averaged over 100 years) than CO
2 as a greenhouse gas; this would immensely magnify the greenhouse effect.
Permafrost
Methane that gets frozen in permafrost – land that is frozen for several years at a time – is slowly released from bogs as the permafrost melts. With rising global temperatures, the amount of permafrost melting and releasing methane continues to increase.
Although records of permafrost are limited, recent years (1999 to 2007) have seen record thawing of permafrost in Alaska and Siberia. Recent measurements in Siberia show that the methane released is five times greater than previously estimated.[20] Melting yedoma, a type of permafrost, is a significant source of atmospheric methane (about 4 Tg of CH4 per year).
The Woods Hole Research Center, citing two 2015 studies on permafrost carbon says there may be a self-reinforcing tipping point where an estimated equivalent of 205 gigatons of carbon dioxide in the form of methane could cause up to 0.5 °C (up to 0.9 °F) warming by the end of the century, which would trigger more warming. Permafrost contains almost twice as much carbon as is present in the atmosphere. The Intergovernmental Panel on Climate Change does not adequately account for arctic methane in permafrost.[21]
Anthropogenic sources of atmospheric methane
Slightly over half of the total emission is due to human activity.[8] Since the Industrial Revolution humans have had a major impact on concentrations of atmospheric methane.
Ecological conversion
Conversion of forests and natural environments into agricultural plots increases the amount of nitrogen in the soil, which inhibits methane oxidation, weakening the ability of the methanotrophic bacteria in the soil to act as sinks. Additionally, by changing the level of the water table, humans can directly affect the soil’s ability to act as a source or sink. The relationship between water table levels and methane emission is explained in the wetlands section of natural sources.
Farm animals
A 2006 UN FAO report reported that livestock generate more greenhouse gases as measured in CO2 equivalents than the entire transportation sector. Livestock accounts for 9 percent of anthropogenic CO2, 65 percent of anthropogenic nitrous oxide and 37 percent of anthropogenic methane. A senior UN official and co-author of the report, Henning Steinfeld, said "Livestock are one of the most significant contributors to today's most serious environmental problems."[22]
Recent NASA research has confirmed the vital role of enteric fermentation in livestock on global warming. "We understand that other greenhouse gases apart from carbon dioxide are important for climate change today," said Gavin Schmidt, the lead author of the study and a researcher at NASA's Goddard Institute for Space Studies in New York, NY and Columbia University's Center for Climate Systems Research.[23] Other recent peer reviewed NASA research published in the journal Science has also indicated that the contribution of methane to global warming has been underestimated.[24][25]
Nicholas Stern, the author of the 2006 Stern Review on climate change has stated "people will need to turn vegetarian if the world is to conquer climate change".[26] President of the National Academy of Sciences Ralph Cicerone (an atmospheric scientist), has indicated the contribution of methane by livestock flatulence and eructation to global warming is a "serious topic." Cicerone states "Methane is the second-most-important greenhouse gas in the atmosphere now. The population of beef cattle and dairy cattle has grown so much that methane from cows now is big. This is not a trivial issue."[27]
Approximately 5% of the methane is released via the flatus, whereas the other 95% is released via eructation. Vaccines are under development to reduce the amount introduced through eructation.[28]
Rice agriculture
Due to a continuously growing world population, rice agriculture has become one of the most powerful anthropogenic sources of methane. With warm weather and water-logged soil, rice paddies act like wetlands, but are generated by humans for the purpose of food production. Due to the swamp-like environment of rice fields, this crop alone is responsible for approximately 50-100 million metric tons of methane emission each year.[29] This means that rice agriculture is responsible for approximately 15 to 20 percent of anthropogenic methane emissions.[30] An article written by William F. Ruddiman explores the possibility that methane emissions started to rise as a result of anthropogenic activity 5000 years ago when ancient cultures started to settle and use agriculture, rice irrigation in particular, as a primary food source.[31]
Landfills
Due to the large collections of organic matter and availability of anaerobic conditions, landfills are the third largest source of atmospheric methane in the United States.[32] Even after a landfill is closed, the mass amount of decaying matter continues to emit methane for years. Although the methanotrophic bacteria in the surrounding soil does oxidize some of the methane, approximately 90 percent of the methane produced in landfills escapes through the landfill cover and into the atmosphere.[33]
Waste water treatment
Waste water treatment facilities act to remove organic matter, solids, pathogens, and chemical hazards as a result of human contamination. Methane emission in waste treatment facilities occurs as a result of anaerobic treatments of organic compounds and anaerobic biodegradation of sludge.[34]
Biomass burning
Incomplete burning of both living and dead organic matter results in the emission of methane. While natural wildfires can contribute to methane emissions, the bulk majority of biomass burning occurs as a result of humans- including everything from accidental burnings by civilians to deliberate burnings used to clear out land to biomass burnings occurring as a result of destroying waste.[19]
Natural gas distribution
Methane is a primary component of natural gas, and thus during the production, processing, storage, transmission, and distribution of natural gas, a significant amount of methane is lost into the atmosphere.[34]
According to the EPA Inventory of U.S Greenhouse Gases and Sinks: 1990-2009 report, dated April 2011, methane emissions from oil and natural gas systems make up 3.8 percent of total greenhouse gas emissions in the United States. Methane emissions occur in all sectors of the natural gas industry, from drilling and production, through gathering and processing and transmission, to distribution. These emissions occur through normal operation, routine maintenance, fugitive leaks, system upsets, and venting of equipment. In the oil industry, some underground crude contains natural gas that is entrained in the oil at high reservoir pressures. When oil is removed from the reservoir, associated natural gas is produced.[35][36]
Additionally, the inventory shows that the oil and natural gas industry emitted 624 billion cubic feet (Bcf) of methane in 2009. Of this amount, 63% was from production operations, 7% from processing, 18% from transmission and storage systems, and 12% from distribution systems. Oil and natural gas systems are the largest human-made source of methane emissions (37 percent) in the United States.[35][36]
Similarly, according to the EPA Global Anthropogenic Non CO2 Greenhouse Gas Emissions: 1990-2020 report, dated June 2006, oil and natural gas operations are a significant source of global methane emissions, constituting approximately 18 percent of total human-made methane emissions. The report also indicates that in 2005, global oil and natural gas methane emissions totaled approximately 82 billion cubic meters (Bcm), or 2,896 billion cubic feet (Bcf), equivalent to about 1,165 million tonnes carbon dioxide equivalent (MtCO2e).[36][37]
Recent estimates of natural gas losses during distribution are as high as 9%[38]
Coal mining
In 2014 NASA researchers reported the discovery of a 2,500 square miles (6,500 km2) methane cloud floating over the Four Corners region of the south-west United States. The discovery was based on data from the European Space Agency’s Scanning Imaging Absorption Spectrometer for Atmospheric Chartography instrument from 2002 to 2012.[39]
The report concluded that "the source is likely from established gas, coal, and coalbed methane mining and processing." The region emitted 590,000 metric tons of methane every year between 2002 and 2012—almost 3.5 times the widely used estimates in the European Union’s Emissions Database for Global Atmospheric Research.[39]
Removal processes
Any process that consumes methane from the atmosphere can be considered a "sink" of atmospheric methane. The most prominent of these processes occur as a result of methane either being destroyed in the atmosphere or broken down in soil.
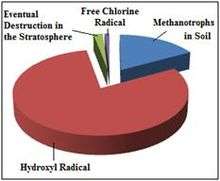
Reaction with the hydroxyl radical- The major removal mechanism of methane from the atmosphere involves radical chemistry; it reacts with the hydroxyl radical (·OH) in the troposphere or stratosphere to create the CH·3 radical and water vapor. In addition to being the largest known sink for atmospheric methane, this reaction is one of the most important sources of water vapor in the upper atmosphere.
4 + ·OH → ·CH
3 + H
2O
This reaction in the troposphere gives a methane lifetime of 9.6 years. Two more minor sinks are soil sinks (160 year lifetime) and stratospheric loss by reaction with ·OH, ·Cl and ·O1D in the stratosphere (120 year lifetime), giving a net lifetime of 8.4 years.[11] Oxidation of methane is the main source of water vapor in the upper stratosphere (beginning at pressure levels around 10 kPa).
The methyl radical formed in the above reaction will, during normal daytime conditions in the troposphere, usually react with another hydroxyl radical to form formaldehyde. Note that this is not strictly oxidative pyrolysis as described previously. Formaldehyde can react again with a hydroxyl radical to form carbon dioxide and more water vapor. Sidechains in these reactions may interact with nitrogen compounds that will likely produce ozone, thus supplanting radicals required in the initial reaction.[40]
Methanotrophic bacteria in soils- Methanotrophic bacteria that reside within soil use methane as a source of carbon in methane oxidation.[41] Methane oxidation allows methanotrophic bacteria to use methane as a source of energy, reacting methane with oxygen and as a result producing carbon dioxide and water.
CH4 + 2O2→ CO2 + 2H2O
Natural sinks of atmospheric methane
Most natural sinks occur as a result of chemical reactions in the atmosphere as well as oxidation by methane consuming bacteria in Earth’s soils.
Methanotrophs in soils
Soils act as a major sink for atmospheric methane through the methanotrophic bacteria that reside within them. This occurs with two different types of bacteria. "High capacity-low affinity" methanotrophic bacteria grow in areas of high methane concentration, such as waterlogged soils in wetlands and other moist environments. And in areas of low methane concentration, "low capacity-high affinity" methanotrophic bacteria make use of the methane in the atmosphere to grow, rather than relying on methane in their immediate environment.[41]
Forest soils act as good sinks for atmospheric methane because soils are optimally moist for methanotroph activity, and the movement of gases between soil and atmosphere (soil diffusivity) is high.[41] With a lower water table, any methane in the soil has to make it past the methanotrophic bacteria before it can reach the atmosphere.
Wetland soils, however, are often sources of atmospheric methane rather than sinks because the water table is much higher, and the methane can be diffused fairly easily into the air without have to compete with the soil’s methanotrophs.
Troposphere
The most effective sink of atmospheric methane is the hydroxyl radical in the troposphere, or the lowest portion of Earth’s atmosphere. As methane rises into the air, it reacts with the hydroxyl radical to create water vapor and carbon dioxide. The lifespan of methane in the atmosphere was estimated at 9.6 years as of 2001; however, increasing emissions of methane over time reduce the concentration of the hydroxyl radical in the atmosphere.[19] With less OH˚ to react with, the lifespan of methane could also increase, resulting in greater concentrations of atmospheric methane.
Stratosphere
Even if it is not destroyed in the troposphere, methane can usually only last 12 years before it is eventually destroyed in Earth’s next atmospheric layer: the stratosphere. Destruction in the stratosphere occurs the same way that it does in the troposphere: methane is oxidized to produce carbon dioxide and water vapor.
Reaction with free chlorine
Methane also reacts with natural chlorine gas in the atmosphere to produce chloromethane and hydrochloric acid (HCl). This process is known as free radical halogenations.[42]
CH4 + Cl2 → CH3Cl + HCl
Anthropogenic sinks of atmospheric methane
Humans have yet to act as any significant sink of atmospheric methane.
Patterns of methane change over time
Since the 1800s, atmospheric methane concentrations have increased annually at a rate of about 0.9%.[12] Long term atmospheric measurements of methane by NOAA show that the build up of methane has slowed dramatically over the last decade, after nearly tripling since pre-industrial times.[43] Although scientists have yet to pinpoint the exact reason(s) for this sudden drop in growth rates, it is thought that this reduction is due to reduced industrial emissions and drought in wetland areas.
The only exceptions to this drop in growth rate occurred in 1991 and 1998 when growth rates increased suddenly to 14-15 nmol/mol per year for those years, nearly double the growth rates of the years before.[14]
The 1991 spike is believed to have occurred due to the volcanic eruption of Mt. Pinatubo in June of that year. Volcanoes affect atmospheric methane emissions when they erupt, releasing ash and sulfur dioxide into the air. As a result, photochemistry of plants is affected and the removal of methane via the tropospheric hydroxyl radical is reduced. However, growth rates quickly fell due to lower temperatures and global reduction in rainfall.
The cause of the 1998 spike is unresolved, but scientists are currently attributing it to a combination of increased wetland and rice field emissions as well as an increased amount of biomass burning. 1998 was also the warmest year since surface temperatures were first recorded, suggesting that anomalously high temperatures can induce elevated methane emission.[44]
Data from 2007 suggested methane concentrations were beginning to rise again.[45] This was confirmed in 2010 when a study showed methane levels were on the rise for the 3 years 2007 to 2009. After a decade of near-zero growth in methane levels, "globally averaged atmospheric methane increased by [approximately] 7 nmol/mol per year during 2007 and 2008. During the first half of 2009, globally averaged atmospheric CH4 was [approximately] 7 nmol/mol greater than it was in 2008, suggesting that the increase will continue in 2009."[46]
Methane emissions levels vary greatly depending on the local geography. For both natural and anthropogenic sources, higher temperatures and higher water levels result in the anaerobic environment that is necessary for methane production.
Natural methane cycles
Emissions of methane into the atmosphere are directly related to temperature and moisture. Thus, the natural environmental changes that occur during seasonal change act as a major control of methane emission. Additionally, even changes in temperature during the day can affect the amount of methane that is produced and consumed.
For example, plants that produce methane can emit as much as two to four times more methane during the day than during the night.[12] This is directly related to the fact that plants tend to rely on solar energy to enact chemical processes.
Additionally, methane emissions are affected by the level of water sources. Seasonal flooding during the spring and summer naturally increases the amount of methane released into the air.
Changes in anthropogenic sources
The most clearly identified rise in atmospheric methane as a result of human activity occurred in the 1700s during the industrial revolution. As technology increased at a considerable rate, humans began to build factories and plants, burn fossil fuels for energy, and clear out forests and other vegetation for the purpose of building and agriculture. This growth continued to rise at a rate of almost 1 percent per year until around 1990 when growth rates dropped to almost zero.[14]
A recent article from William F. Ruddiman, however, indicates that the anthropogenic change in methane may have started 5000 years prior to the industrial revolution.[31] The methane insolation cycles of the ice core remained stable and predictable until 5000 years ago, most likely due to some anthropogenic effect.[31] Ruddiman suggests that the transition of humans from hunter gatherers into agricultural farming was the first instance of humans affecting methane concentration in the atmosphere. Ruddiman’s hypothesis is supported by the fact that early rice irrigation occurred approximately 5000 years ago—the same time the ice core cycles lost their predictability. Due to the inefficiency of humans first learning how to grow rice, extensive rice paddies would have been needed to feed even a small population. These, over-flooded and filled with weeds, would have resulted in huge methane emitting wetlands.[31]
Another source of methane emissions has been identified in Russia. Near Yamburg and Urengoy exist gas fields with a methane concentration of 97 percent.[47] The gas obtained from these fields is taken and exported to Western and Central Europe through an extensive pipeline system known as the Trans-Siberian natural gas pipeline system. In accordance with the IPCC and other natural gas emissions control groups, measurements had to be taken throughout the pipeline to measure methane emissions from technological discharges and leaks at the pipeline fittings and vents. Although the majority of the natural gas leaks were carbon dioxide, a significant amount of methane was also being consistently released from the pipeline as a result of leaks and breakdowns. In 2001, natural gas emissions from the pipeline and natural gas transportation system accounted for 1 percent of the natural gas produced.[47] Fortunately, between 2001 and 2005, this number reduced to 0.7 percent, and even the 2001 value is still significantly less than that of 1996.[47] Thus, it is suggested that while natural gas transportation is a significant anthropogenic source of methane, over time as technology advances and greenhouse gas emission awareness increases, methane emission growth rates decrease and natural gases are overall better managed and controlled.
However that may be, transportation is only part of the problem. Indeed, Howarth [48] et al. have argued that:
We believe the preponderance of evidence indicates shale gas has a larger GHG [green house gas] footprint than conventional gas, considered over any time scale. The GHG footprint of shale gas also exceeds that of oil or coal when considered at decadal time scales, […]
For more recent work confirming these results see Howarth's "A bridge to nowhere: methane emissions and the greenhouse gas footprint of natural gas,"[49] and "Methane emissions and climatic warming risk from hydraulic fracturing and shale gas development: implications for policy."[50]
As a recent study [51] by Miller et al. shows, current greenhouse gas reduction policies in the US are based on what appear to be significant underestimates of anthropogenic methane emissions. As the authors state:
We find greenhouse gas emissions from agriculture and fossil fuel extraction and processing (i.e., oil and/or natural gas) are likely a factor of two or greater than cited in existing studies.
Impacts
The direct radiative greenhouse gas forcing effect has been estimated at 0.5 W/m2.[52]
Methane is a strong GHG with a global warming potential 86 times greater than CO2 in a 20-year time frame; Methane is not as persistent a gas and tails off to about 29 for a 100 year time frame.[53]
In addition to the direct heating effect and the normal feedbacks, the methane breaks down to carbon dioxide and water. This water is often above the tropopause where little water usually reaches. Ramanathan (1988)[54] notes that both water and ice clouds, when formed at cold lower stratospheric temperatures, are extremely efficient in enhancing the atmospheric greenhouse effect. He also notes that there is a distinct possibility that large increases in future methane may lead to a surface warming that increases nonlinearly with the methane concentration.
Ozone layer
Methane also affects the degradation of the ozone layer, when methane is transformed into water in the stratosphere. This process is enhanced by global warming, because warmer air holds more water vapor than colder air, so the amount of water vapor in the atmosphere increases as it is warmed by the greenhouse effect. Climate models also indicate that greenhouse gases such as carbon dioxide and methane may enhance the transport of water into the stratosphere; though this is not fully understood.[55]
Methane management techniques
In an effort to mitigate climate change, humans have started to develop alternative methods and medicines.
For example, in order to counteract the immense amount of methane that ruminants give off, a type of drug called monensin (marketed as rumensin™) has been developed. This drug is classified as an ionophore, which is an antibiotic that is naturally produced by a harmless bacteria strain. This drug not only improves feed efficiency but also reduces the amount of methane gas emitted from the animal and its manure.[56]
In addition to medicine, specific manure management techniques have been developed to counteract harmful emissions from livestock manure. Educational resources have even begun to be provided for small farms run by owners who do not realize the harmful effects of livestock manure on the environment. Management techniques include daily pickup and storage of manure in a completely closed off storage facility that will prevent runoff from making it into bodies of water. The manure can then be kept in storage until it is either reused for fertilizer or taken away and stored in an offsite compost. Nutrient levels of various animal manures are even provided for optimal use as compost for gardens and agriculture.[57]
In order to reduce effects on methane oxidation in soil, several steps can be taken. Controlling the usage of nitrogen enhancing fertilizer and reducing the amount of nitrogen pollution into the air can both lower inhibition of methane oxidation—a major sink of atmospheric methane. Additionally, using drier growing conditions for crops such as rice and selecting strains of crops that produce more food per unit area can reduce the amount of land with ideal conditions for methanogenesis. Careful selection of areas of land conversion (for example, plowing down forests to create agricultural fields) can also reduce the destruction of major areas of methane oxidation.
To counteract methane emissions from landfills, on March 12, 1996, the EPA (Environmental Protection Agency) added the "Landfill Rule" to the Clean Air Act. This rule requires large landfills that have ever accepted municipal solid waste, have been used as of November 8, 1987, can hold at least 2.5 million metric tons of waste with a volume greater than 2.5 million cubic meters, and/or have nonmethane organic compound (NMOC) emissions of at least 50 metric tons per year to collect and combust emitted landfill gas.[33] This set of requirements excludes 96% of the landfills in the USA. While the direct result of this is landfills reducing emission of nonmethane compounds that form smog, the indirect result is reduction of methane emissions as well.
To reduce emissions from the natural gas industries, the EPA developed the Natural Gas STAR Program, also known as Gas STAR.[34]
Another program was also developed by the EPA to reduce emissions from coal mining. The Coalbed Methane Outreach Program (CMOP) helps and encourages the mining industry to find ways to use or sell methane that would otherwise be released from the coal mine into the atmosphere.[34]
A portable methane detector has been developed which, mounted in a vehicle, can detect excess levels of methane in the ambient atmosphere and differentiate between natural methane from rotting vegetation or manure and gas leaks. As of 2013 the technology was being deployed by Pacific Gas & Electric.[58]
See also
References
- ↑ IPCC AR5 WG1 (2013). "Climate Change 2013: The Physical Science Basis - Anthropogenic and Natural Radiative Forcing Supplementary Material" (PDF). Cambridge University Press.
- ↑ Drew T. Shindell*, Greg Faluvegi, Dorothy M. Koch, Gavin A. Schmidt, Nadine Unger, Susanne E. Bauer (2009) (in German), Improved attribution of climate forcing to emissions (Science 326 ed.), AAAS, pp. 716–718, doi:10.1126/science.1174760 Online
- ↑ IPCC AR5 WG1 (2013). "Climate Change 2013: The Physical Science Basis - Summary for Policymakers" (PDF). Cambridge University Press.
- ↑ Gale, Joseph (2009). Astrobiology of Earth : the emergence, evolution, and future of life on a planet in turmoil. Oxford: Oxford University Press. ISBN 978-0-19-920580-6.
- ↑ Multiple (2001). "Climate Change 2001: Working Group I: The Scientific Basis". Technical Summary. Intergovernmental Panel on Climate Change (IPCC). p. 6. Retrieved 6 March 2015.
- ↑ Multiple authors (June 1988). "Historical CO2 record derived from a spline fit (20 year cutoff) of the Law Dome DE08 and DE08-2 ice cores". Multiple works. Retrieved 6 March 2015.
- ↑ Phys.org - methane
- 1 2 "Technical summary". Climate Change 2001. United Nations Environment Programme.
- ↑ "Is Canada's forest a carbon sink or source?". Natural Resources Canada. Retrieved 21 March 2013.
- ↑ GMAO Chemical Forecasts and GEOS–CHEM NRT Simulations for ICARTT (top) and Randy Kawa, NASA GSFC Atmospheric Chemistry and Dynamics Branch (lower).
- 1 2 "Trace Gases: Current Observations, Trends, and Budgets". Climate Change 2001, IPCC Third Assessment Report. IPCC/United Nations Environment Programme.
- 1 2 3 Bubier, Jill L.; Moore, Tim R. "An ecological perspective on methane emissions from northern wetlands". Trends in Ecology an Evolution.
- ↑ Margonelli, Lisa (September 2008). "Gut Reactions". The Atlantic. Retrieved 16 January 2012.
- 1 2 3 4 "Ch.2 Changes in Atmospheric Constituents and in Radiative Forcing". Climate Change 2007 IPCC Fourth Assessment Report. IPPC.
- ↑ Keppler, Frank; Hamilton, John T. G.; Brass, Marc; Rockman, Thomas (2005-11-03). "Methane emissions from terrestrial plants under aerobic conditions". Nature (Nature Publishing Group) 439 (7073): 187–191. Bibcode:2006Natur.439..187K. doi:10.1038/nature04420. ISSN 0028-0836. PMID 16407949. Archived from the original on 15 January 2010. Retrieved 2010-01-20.
- ↑ Hirsch, Tim (2006-01-11). "Plants revealed as methane source". BBC News. Archived from the original on 13 October 2006. Retrieved 2006-09-07.
- ↑ Keppler, Frank; Hamilton, John T. G.; Brass, Marc; Rockman, Thomas (2006-01-18). "Global warming - the blame is not with the plants". EurekAlert! (American Association for the Advancement of Science). Archived from the original on 1 September 2006. Retrieved 2006-09-06.
- ↑ Duek, Tom A.; Ries de Visser; Hendrik Poorter; Stefan Persijn; Antonie Gorissen; Willem de Visser; Ad Schapendonk; Jan Verhagen; Jan Snel; Frans J. M. Harren; Anthony K. Y. Ngai; Francel Verstappen; Harro Bouwmeester; Laurentius A. C. J. Voesenek; Adrie van der Werf (2007-03-30). "No evidence for substantial aerobic methane emission by terrestrial plants: a 13C-labelling approach.". New Phytologist (Blackwell) 175 (1): 29–35. doi:10.1111/j.1469-8137.2007.02103.x. PMID 17547664. Retrieved 2007-04-23.
- 1 2 3 "Methane and Nitrous Oxide Emissions From Natural Sources" (PDF). USA Environmental Protection Agency Office of Atmospheric Programs. April 2010.
- ↑ "Methane bubbles climate trouble". BBC News. 2006-09-07. Retrieved 2006-09-07.
- ↑ Abraham, John (13 October 2015). "Methane release from melting permafrost could trigger dangerous global warming". Newspaper. The Guardian. Retrieved 13 October 2015.
- ↑ "Livestock a major threat to environment". United Nations Food and Agriculture Organization. 29 November 2006. Retrieved 4 November 2011.
- ↑ "Methane Explosion Warmed the Prehistoric Earth". NASA GISS: Research News. 2010-12-10. Retrieved 2011-11-03.
- ↑ Shindell, 2 Greg; Faluvegi, G.; Koch, Dorothy M.; Schmidt, Gavin A.; Unger, Nadine; Bauer, Susanne E. (30 October 2009). "Improved Attribution of Climate Forcing to Emissions". Science 326 (5953): 716–718. Bibcode:2009Sci...326..716S. doi:10.1126/science.1174760. PMID 19900930. Retrieved 4 November 2011.
- ↑ Vergano, Dan (2009-10-29). "Methane's role in global warming underestimated". USA Today.
- ↑ Pagnamenta, Robin (2009-10-27). "Climate chief Lord Stern give up meat to save the planet". The Times (London).
- ↑ Gary Polakovic (7 June 2003). "Getting the Cows to Cool It". The Los Angeles Times. Retrieved 4 November 2011.
- ↑ Rachel Nowak (25 September 2004). "Burp vaccine cuts greenhouse gas emissions". New Scientist. Retrieved 4 November 2011.
- ↑ "Methane Sources - Rice Paddies". GreenHouse Gas Online.org. 2008. Retrieved 11 November 2011.
- ↑ "Methane emission and rice agriculture." http://www.ias.ac.in/currsci/aug252001/345.pdf
- 1 2 3 4 Ruddiman, William F. "The Anthropogenic Greenhouse Era Began Thousands of Years Ago."
- ↑ "Greenhouse Gas Emissions". United States Environmental Protection Agency. Retrieved 21 March 2013.
- 1 2 "Landfill Methane Energy Recovery." http://www.uspowerpartners.org/Topics/SECTION6Topic-LandfillMethane.htm
- 1 2 3 4 "Sources and Emissions." http://www.epa.gov/methane/sources.html
- 1 2 "U.S. INVENTORY OF U.S. GREENHOUSE GAS EMISSIONS AND SINKS: 1990-2010 (April 2012)". Greenhouse Gas Inventory Report. United States Environmental Protection Agency. April 2012. Retrieved February 20, 2013.
- 1 2 3 "How much methane is emitted from oil and natural gas systems? What are the major emission sources?". Frequent Questions Natural Gas STAR Program. United States Environmental Protection Agency. Last updated on 05/24/2012. Retrieved February 20, 2013. Check date values in:
|date=(help) - ↑ "Global Anthropogenic Non-co2 Greenhouse Gas Emissions 1990-2020". United States Environmental Protection Agency. June 2006. EPA Document number 430R06003. Retrieved February 20, 2013.
- ↑ Tollafson, j (January 2013). "Methane leaks erode green credentials of natural gas" (PDF). Nature 493: 12. Bibcode:2013Natur.493...12T. doi:10.1038/493012a. Retrieved 1 Oct 2014.
- 1 2 Gass, Henry (October 10, 2014). "How scientists overlooked a 2,500-square-mile cloud of methane over the Southwest". Christian Science Monitor. Retrieved October 24, 2014.
- ↑ Loïc Jounot (2006). "Tropospheric Chemistry". University of Toronto Atmospheric Physics Department. Archived from the original on 17 June 2008. Retrieved 2008-07-18.
- 1 2 3 "Methane Sinks—Soils." http://www.ghgonline.org/methanesinksoil.htm
- ↑ Clark, Jim. 2000. "Explaining the Reaction between Methane and Chlorine." http://www.chemguide.co.uk/mechanisms/freerad/ch4andcl2tt.html
- ↑ "Scientists pinpoint cause of slowing methane emissions". National Oceanic & Atmospheric Administration news Online. 2006-09-28. Archived from the original on 26 May 2007. Retrieved 2007-05-23.
- ↑ Denman, K.L.; et al. "7. Couplings Between Changes in the Climate System and Biogeochemistry.". IPCC AR4 WG1 2007. Retrieved 2011-11-04.
- ↑ "Annual Greenhouse Gas Index (AGGI) Indicates Sharp Rise in Carbon Dioxide and Methane in 2007". National Oceanic & Atmospheric Administration - Earth System Research Laboratory. 2008-04-23. Retrieved 2008-06-16.
- ↑ Heidi Blake (February 22, 2010). "Climate change could be accelerated by 'methane time bomb'". The Telegraph.
- 1 2 3 Wuppertal and Mainz. 2005. " Greenhouse Gas Emissions from the Russian Natural Gas Export Pipeline System." http://www.apat.gov.it/site/_files/Greenhouse_Gas.pdf
- ↑ http://www.eeb.cornell.edu/howarth/Howarthetal2012_Final.pdf
- ↑ http://onlinelibrary.wiley.com/doi/10.1002/ese3.35/pdf
- ↑ https://www.dovepress.com/methane-emissions-and-climatic-warming-risk-from-hydraulic-fracturing--peer-reviewed-article-EECT
- ↑ http://www.pnas.org/cgi/doi/10.1073/pnas.1314392110
- ↑ "AR4 Fig 2.4". Climate Change 2007. United Nations Environment Programme.
- ↑ Intergovernmental Panel on Climate Change. Summary for policymakers. in: Climate change 2013: The physical science basis. contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change. Technical report, Intergovernmental Panel on Climate Change, Cambridge, United Kingdom and New York, NY, USA., 2013
- ↑ "Ramanathan". Trace-Gas Greenhouse Effect and Global Warming: Underlying Principles and Outstanding Issues. Ambio-Royal Swedish Academy of sciences.
- ↑ Drew Shindell (2001). "Wetter Upper Atmosphere May Delay Global Ozone Recovery". NASA.
- ↑ Use of Rumensin in Dairy Diets." http://www.extension.org/pages/Use_of_Rumensin_in_Dairy_Diets
- ↑ Bradley, Athena Lee. Northeast Recycling Councel, Inc. 2008. "Manure Management for Small and Hobby Farms." http://www.nerc.org/documents/manure_management/manure_management_handbook.pdf
- ↑ Matthew L. Wald (August 6, 2013). "New Tools Pinpoint Natural Gas Leaks, Maximizing a Fuel’s Green Qualities". The New York Times. Retrieved August 7, 2013.
External links
- Methane in tundra and oceans to be released in atmosphere
- Methane hydrate stability and anthropogenic climate change Biogeosciences Discuss., 4, 993-1057, 2007
- Methane: A Scientific Journey from Obscurity to Climate Super-Stardom Good Sept. 2004 background report from NASA Goddard Institute for Space Studies (GISS)