Ascorbic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one | |
| Other names
Vitamin C | |
| Identifiers | |
| 50-81-7 | |
| ChEBI | CHEBI:29073 |
| ChEMBL | ChEMBL196 |
| ChemSpider | 10189562 |
| EC Number | 200-066-2 |
| 4781 | |
| Jmol interactive 3D | Image Image |
| KEGG | D00018 |
| PubChem | 5785 |
| UNII | PQ6CK8PD0R |
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.12 g·mol−1 |
| Appearance | White or light yellow solid |
| Density | 1.65 g/cm3 |
| Melting point | 190 to 192 °C (374 to 378 °F; 463 to 465 K) decomposes |
| 330 g/L | |
| Solubility in ethanol | 20 g/L |
| Solubility in glycerol | 10 g/L |
| Solubility in propylene glycol | 50 g/L |
| Solubility in other solvents | insoluble in diethyl ether, chloroform, benzene, petroleum ether, oils, fats |
| Acidity (pKa) | 4.10 (first), 11.6 (second) |
| Pharmacology | |
| ATC code | A11 G01AD03, S01XA15 |
| Hazards | |
| Safety data sheet | JT Baker Oxford University |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose) |
11.9 g/kg (oral, rat)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form ("vitamer") of vitamin C. It was originally called L-hexuronic acid, but, when it was found to have vitamin C activity in animals ("vitamin C" being defined as a vitamin activity, not then a specific substance), the suggestion was made to rename it. The new name, ascorbic acid, is derived from a- (meaning "no") and scorbutus (scurvy), the disease caused by a deficiency of vitamin C. Because it is derived from glucose, many non-human animals are able to produce it, but humans require it as part of their nutrition. Other vertebrates which lack the ability to produce ascorbic acid include some primates, guinea pigs, teleost fishes, bats, and some birds, all of which require it as a dietary micronutrient (that is, in vitamin form).[2]
History
From the middle of the 18th century, it was noted that lemon and lime juice could help prevent sailors from getting scurvy. At first, it was supposed that the acid properties were responsible for this benefit; however, it soon became clear that other dietary acids, such as vinegar, had no such benefits. In 1907, two Norwegian physicians reported an essential disease-preventing compound in foods that was distinct from the one that prevented beriberi. These physicians were investigating dietary-deficiency diseases using the new animal model of guinea pigs, which are susceptible to scurvy. The newly discovered food-factor was eventually called vitamin C.
From 1928 to 1932, the Hungarian research team led by Albert Szent-Györgyi, as well as that of the American researcher Charles Glen King, identified the antiscorbutic factor as a particular single chemical substance. At the Mayo clinic, Szent-Györgyi had isolated the chemical hexuronic acid from animal adrenal glands. He suspected it to be the antiscorbutic factor but could not prove it without a biological assay. This assay was finally conducted at the University of Pittsburgh in the laboratory of King, which had been working on the problem for years, using guinea pigs. In late 1931, King's lab obtained adrenal hexuronic acid indirectly from Szent-Györgyi and, using their animal model, proved that it is vitamin C, by early 1932.
This was the last of the compound from animal sources, but, later that year, Szent-Györgyi's group discovered that paprika pepper, a common spice in the Hungarian diet, is a rich source of hexuronic acid. He sent some of the now-more-available chemical to Walter Norman Haworth, a British sugar chemist.[3] In 1933, working with the then-Assistant Director of Research (later Sir) Edmund Hirst and their research teams, Haworth deduced the correct structure and optical-isomeric nature of vitamin C, and in 1934 reported the first synthesis of the vitamin.[4] In honor of the compound's antiscorbutic properties, Haworth and Szent-Györgyi now proposed the new name of "a-scorbic acid" for the compound. It was named L-ascorbic acid by Haworth and Szent-Györgyi when its structure was finally proven by synthesis.[5]
In 1937, the Nobel Prize for chemistry was awarded to Haworth for his work in determining the structure of ascorbic acid — shared with Paul Karrer, who received his award for work on vitamins — and the prize for Physiology or Medicine that year went to Albert Szent-Györgyi for his studies of the biological functions of L-ascorbic acid.
The American physician Fred R. Klenner, M.D. promoted vitamin C as a cure for many diseases in the 1950s by elevating the dosages greatly to as much as tens of grams vitamin C daily orally and by injection. From 1967 on, Nobel prize winner Linus Pauling recommended high doses of ascorbic acid as a prevention against cold and cancer. However, modern evidence does not support a role for high dose vitamin C in the treatment of cancer or the prevention of the common cold in the general population.[6][7]
Acidity
 Canonical structures for the ascorbate anion
Canonical structures for the ascorbate anion
Ascorbic acid is classed as a reductone. The ascorbate anion is stabilized by electron delocalization, as shown above in terms of resonance between two canonical forms. For this reason, ascorbic acid is much more acidic than would be expected if the compound contained only isolated hydroxyl groups.
Antioxidant mechanism

The ascorbate ion is the predominant species at typical biological pH values. It is a mild reducing agent and antioxidant. It is oxidized with loss of one electron to form a radical cation and then with loss of a second electron to form dehydroascorbic acid. It typically reacts with oxidants of the reactive oxygen species, such as the hydroxyl radical. Such radicals are damaging to animals and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids. Sometimes these radicals initiate chain reactions. Ascorbate can terminate these chain radical reactions by electron transfer. Ascorbic acid is special because it can transfer a single electron, owing to the resonance-stabilized nature of its own radical ion, called semidehydroascorbate. The net reaction is:
- RO • + C6H7O6− → RO- + C6H7O6• → → ROH + C6H6O6[8]
The oxidized forms of ascorbate are relatively unreactive and do not cause cellular damage.
However, being a good electron donor, excess ascorbate in the presence of free metal ions can not only promote but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts.
On exposure to oxygen, ascorbic acid will undergo further oxidative decomposition to various products including diketogulonic acid, xylonic acid, threonic acid and oxalic acid.[9]
Reactions
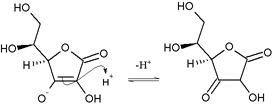 Nucleophilic attack of ascorbic enol on proton to give 1,3-diketone
Nucleophilic attack of ascorbic enol on proton to give 1,3-diketone
Food chemistry
Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives. These compounds are water-soluble and, thus, cannot protect fats from oxidation: For this purpose, the fat-soluble esters of ascorbic acid with long-chain fatty acids (ascorbyl palmitate or ascorbyl stearate) can be used as food antioxidants. Eighty percent of the world's supply of ascorbic acid is produced in China.[10]
The relevant European food additive E numbers are:
- E300 ascorbic acid (approved for use as a food additive in the EU[11] USA[12] and Australia and New Zealand)[13]
- E301 sodium ascorbate (approved for use as a food additive in the EU[11] USA[14] and Australia and New Zealand)[13]
- E302 calcium ascorbate (approved for use as a food additive in the EU[11] USA[12] and Australia and New Zealand)[13]
- E303 potassium ascorbate
- E304 fatty acid esters of ascorbic acid (i) ascorbyl palmitate (ii) ascorbyl stearate.
It creates volatile compounds when mixed with glucose and amino acids in 90 °C.[15]
It is a cofactor in tyrosine oxidation.[16]
Niche, non-food uses
- Ascorbic acid is easily oxidized and so is used as a reductant in photographic developer solutions (among others) and as a preservative.
- In fluorescence microscopy and related fluorescence-based techniques, ascorbic acid can be used as an antioxidant to increase fluorescent signal and chemically retard dye photobleaching.[17]
- It is also commonly used to remove dissolved metal stains, such as iron, from fiberglass swimming pool surfaces.
- In plastic manufacturing, ascorbic acid can be used to assemble molecular chains more quickly and with less waste than traditional synthesis methods.[18]
- Heroin users are known to use ascorbic acid as a means to convert heroin base to a water-soluble salt so that it can be injected.[19]
- As justified by its reaction with iodine, it is used to negate the effects of iodine tablets in water purification. It reacts with the sterilized water, removing the taste, color, and smell of the iodine. This is why it is often sold as a second set of tablets in most sporting goods stores as Portable Aqua-Neutralizing Tablets, along with the potassium iodide tablets.
- Intravenous high-dose ascorbate is being used as a chemotherapeutic and biological response modifying agent.[20] Currently it is still under clinical trials.[21]
Biosynthesis
Ascorbic acid is found in plants and animals where it is produced from glucose.[22] Animals must either produce it or digest it, otherwise a lack of vitamin C may cause scurvy, which may eventually lead to death. Reptiles and older orders of birds make ascorbic acid in their kidneys. Recent orders of birds and most mammals make ascorbic acid in their liver where the enzyme L-gulonolactone oxidase is required to convert glucose to ascorbic acid.[22] Humans, other higher primates, guinea pigs and most bats require dietary L-gulonolactone oxidase because the enzyme catalysing the last step in the biosynthesis is highly mutated and non-functional, therefore, unable to make ascorbic acid. Synthesis and signalling properties are still under investigation.[23]
Animal ascorbic acid biosynthesis pathway
The biosynthesis of ascorbic acid starts with the formation of UDP-glucuronic acid. UDP-glucuronic acid is formed when UDP-glucose undergoes two oxidations catalyzed by the enzyme UDP-glucose 6-dehydrogenase. UDP-glucose 6-dehydrogenase uses the co-factor NAD+ as the electron acceptor. The transferase UDP-glucuronate pyrophosphorylase removes a UMP and glucuronokinase, with the cofactor ADP, removes the final phosphate leading to D-glucuronic acid. The aldehyde group of this is reduced to a primary alcohol using the enzyme glucuronate reductase and the cofactor NADPH, yielding L-gulonic acid. This is followed by lactone formation with the hydrolase gluconolactonase between the carbonyl on C1 and hydroxyl group on the C4. L-Gulonolactone then reacts with oxygen, catalyzed by the enzyme L-gulonolactone oxidase (which is nonfunctional in humans and other Haplorrhini primates) and the cofactor FAD+. This reaction produces 2-oxogulonolactone, which spontaneously undergoes enolization to form ascorbic acid.[24]
Plant ascorbic acid biosynthesis pathway
There are many different biosynthesis pathways for ascorbic acid in plants. Most of these pathways are derived from products found in glycolysis and other pathways. For example, one pathway goes through the plant cell wall polymers.[23] The plant ascorbic acid biosynthesis pathway most principal seems to be L-galactose. L-Galactose reacts with the enzyme L-galactose dehydrogenase, whereby the lactone ring opens and forms again but with between the carbonyl on C1 and hydroxyl group on the C4, resulting in L-galactonolactone.[24] L-Galactonolactone then reacts with the mitochondrial flavoenzyme L-galactonolactone dehydrogenase.[25] to produce ascorbic acid.[24] L-Ascorbic acid has a negative feedback on L-galactose dehydrogenase in spinach.[26] Ascorbic acid efflux by embryo of dicots plants is a well-established mechanism of iron reduction, and a step obligatory for iron uptake.[27]
Yeasts do not make L-ascorbic acid but rather a similar antioxidant known as D-erythroascorbic acid.[28]
Industrial preparation
Ascorbic acid is prepared in industry from glucose in a method based on the historical Reichstein process. In the first of a five-step process, glucose is catalytically hydrogenated to sorbitol, which is then oxidized by the microorganism Acetobacter suboxydans to sorbose. Only one of the six hydroxy groups is oxidized by this enzymatic reaction. From this point, two routes are available. Treatment of the product with acetone in the presence of an acid catalyst converts four of the remaining hydroxyl groups to acetals. The unprotected hydroxyl group is oxidized to the carboxylic acid by reaction with the catalytic oxidant TEMPO (regenerated by sodium hypochlorite — bleaching solution). Historically, industrial preparation via the Reichstein process used potassium permanganate as the bleaching solution. Acid-catalyzed hydrolysis of this product performs the dual function of removing the two acetal groups and ring-closing lactonization. This step yields ascorbic acid. Each of the five steps has a yield larger than 90%.[29]
A more biotechnological process, first developed in China in the 1960s, but further developed in the 1990s, bypasses the use of acetone-protecting groups. A second genetically modified microbe species, such as mutant Erwinia, among others, oxidises sorbose into 2-ketogluconic acid (2-KGA), which can then undergo ring-closing lactonization via dehydration. This method is used in the predominant process used by the ascorbic acid industry in China, which supplies 80% of world's ascorbic acid.[30] American and Chinese researchers are competing to engineer a mutant that can carry out a one-pot fermentation directly from glucose to 2-KGA, bypassing both the need for a second fermentation and the need to reduce glucose to sorbitol.[31]
There exists a D-ascorbic acid, which does not occur in nature but can be synthesized artificially. It has identical antioxidant properties to L-ascorbic acid yet has far less vitamin C activity (although not quite zero).[32] This fact is taken as evidence that the antioxidant properties of ascorbic acid are only a small part of its effective vitamin activity. To be specific, L-ascorbate is known to participate in many specific enzyme reactions that require the correct enantiomer (L-ascorbate and not D-ascorbate). L-Ascorbic acid has a specific rotation of ![\textstyle[\alpha]_D^{20} = +23](../I/m/d85292039d92c477a2a73b5230a0ac44.png) °.[33]
°.[33]
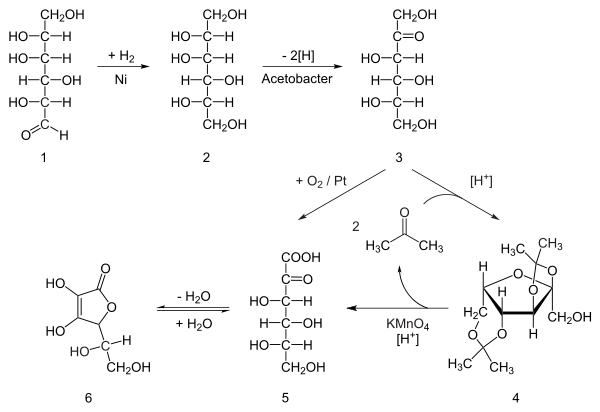
Determination
The traditional way to analyze the ascorbic acid content is the process of titration with an oxidizing agent, and several procedures have been developed, mainly relying on iodometry. Iodine is used in the presence of a starch indicator. Iodine is reduced by ascorbic acid, and, when all the ascorbic acid has reacted, the iodine is then in excess, forming a blue-black complex with the starch indicator. This indicates the end-point of the titration. As an alternative, ascorbic acid can be treated with iodine in excess, followed by back titration with sodium thiosulfate using starch as an indicator.[34] The preceding iodometric method has been revised to exploit reaction of ascorbic acid with iodate and iodide in acid solution. Electrolyzing the solution of potassium iodide produces iodine, which reacts with ascorbic acid. The end of process is determined by potentiometric titration in a manner similar to Karl Fischer titration. The amount of ascorbic acid can be calculated by Faraday's law.
An uncommon oxidising agent is N-bromosuccinimide, (NBS). In this titration, the NBS oxidizes the ascorbic acid in the presence of potassium iodide and starch. When the NBS is in excess (i.e., the reaction is complete), the NBS liberates the iodine from the potassium iodide, which then forms the blue-black complex with starch, indicating the end-point of the titration.
Compendial status
See also
- Colour retention agent
- Erythorbic acid: a diastereomer of ascorbic acid.
- Mineral ascorbates: salts of ascorbic acid
- Acids in wine
Notes and references
- ↑ "Safety (MSDS) data for ascorbic acid". Oxford University. 2005-10-09. Archived from the original on 9 February 2007. Retrieved 2007-02-21.
- ↑ Lachapelle, M. Y.; Drouin, G. (2010). "Inactivation dates of the human and guinea pig vitamin C genes". Genetica 139 (2): 199–207. doi:10.1007/s10709-010-9537-x. PMID 21140195.
- ↑ Story of Vitamin C's chemical discovery. Profiles.nlm.nih.gov. Retrieved on 2012-12-04.
- ↑ Davies, Michael B.; Austin, John; Partridge, David A. (1991). Vitamin C: Its Chemistry and Biochemistry. The Royal Society of Chemistry. p. 48. ISBN 0-85186-333-7.
- ↑ Svirbelf, Joseph Louis; Szent-Györgyi, Albert (April 25, 1932), The Chemical Nature Of Vitamin C (PDF). Part of the National Library of Medicine collection. Accessed January 2007
- ↑ Jacobs, Carmel; Hutton, Brian; Ng, Terry; Shorr, Risa; Clemons, Mark (2015). "Is There a Role for Oral or Intravenous Ascorbate (Vitamin C) in Treating Patients With Cancer? A Systematic Review". The Oncologist 20 (2): 210–223. doi:10.1634/theoncologist.2014-0381.
Conclusion. There is no high-quality evidence to suggest that ascorbate supplementation in cancer patients either enhances the antitumor effects of chemotherapy or reduces its toxicity.
- ↑ "Vitamin C Fact Sheet for Health Professionals". National Institutes of Health. Retrieved 2 June 2015.
"At this time, the evidence is inconsistent on whether dietary vitamin C intake affects cancer risk. Results from most clinical trials suggest that modest vitamin C supplementation alone or with other nutrients offers no benefit in the prevention of cancer... Some researchers support reassessment of the use of high-dose IV vitamin C as a drug to treat cancer... It is uncertain whether supplemental vitamin C and other antioxidants might interact with chemotherapy and/or radiation.
- ↑ R, Caspi (Aug 19, 2009), "MetaCyc Compound: monodehydroascorbate radical", MetaCyc, retrieved 2014-12-08
- ↑ https://books.google.com/books?id=AJW37Xc3uiAC&pg=PA311&dq=decomposition+of+ascorbic+acid
- ↑ Weiss, Rick (May 20, 2007), "Tainted Chinese Imports Common", Washington Post, retrieved 2010-04-25
- 1 2 3 UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- 1 2 US Food and Drug Administration: "Listing of Food Additives Status Part I". Retrieved 2011-10-27.
- 1 2 3 Australia New Zealand Food Standards Code"Standard 1.2.4 – Labelling of ingredients". Retrieved 2011-10-27.
- ↑ US Food and Drug Administration: "Listing of Food Additives Status Part II". Retrieved 2011-10-27.
- ↑ Seck, S.; Crouzet, J. (1981). "Formation of Volatile Compounds in Sugar-Phenylalanine and Ascorbic Acid-Phenylalanine Model Systems during Heat Treatment". Journal of Food Science 46 (3): 790–793. doi:10.1111/j.1365-2621.1981.tb15349.x.
- ↑ Sealock, R. R.; Goodland, R. L.; Sumerwell, W. N.; Brierly, J. M. (1952). "The Role of Ascorbic Acid in the Oxidation of l-Tyrosine by Guinea Pig Liver Extracts" (PDF). Journal of Biological Chemistry 196 (2): 761–767. PMID 12981016.
- ↑ Widengren, J.; Chmyrov, A.; Eggeling, C.; Löfdahl, P.-Å.; Seidel, C. A. (2007). "Strategies to Improve Photostabilities in Ultrasensitive Fluorescence Spectroscopy". The Journal of Physical Chemistry A 111 (3): 429–440. doi:10.1021/jp0646325. PMID 17228891.
- ↑ Vitamin C, water have benefits for plastic manufacturing, Reliable Plant Magazine, 2007, archived from the original on 2007-09-27, retrieved 2007-06-25
- ↑ Beynon, C. M.; McVeigh, J.; Chandler, M.; Wareing, M.; Bellis, M. A. (2007). "The Impact of Citrate Introduction at UK Syringe Exchange Programmes: A Retrospective Cohort Study in Cheshire and Merseyside, UK". Harm Reduction Journal 4 (1): 21. doi:10.1186/1477-7517-4-21. PMC 2245922. PMID 18072971.
- ↑ "The Riordan IVC Protocol for Adjunctive Cancer Care: Intravenous Ascorbate as a Chemotherapeutic and Biological Response Modifying Agent" (PDF). Riordan Clinic Research Institut. February 2013. Retrieved 2 February 2014.
- ↑ "High-Dose Vitamin C (PDQ®): Human/Clinical Studies". National Cancer Institute. Retrieved 2 February 2014.
- 1 2 Stone, Irwin (1972), The Natural History of Ascorbic Acid in the Evolution of Mammals and Primates
- 1 2 Valpuesta, V.; Botella, M. A. (2004). "Biosynthesis of L-Ascorbic Acid in Plants: New Pathways for an Old Antioxidant" (PDF). Trends in Plant Science 9 (12): 573–577. doi:10.1016/j.tplants.2004.10.002. PMID 15564123.
- 1 2 3 Dewick, P. M. (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). John Wiley and Sons. p. 493. ISBN 978-0470741672.
- ↑ Leferink, N. G.; van den Berg, W. A.; van Berkel, W. J. (2008). "L-Galactono-γ-lactone Dehydrogenase from Arabidopsis thaliana, a Flavoprotein Involved in Vitamin C Biosynthesis" (PDF). FEBS Journal 275 (4): 713–726. doi:10.1111/j.1742-4658.2007.06233.x.
- ↑ Mieda, T.; Yabuta, Y.; Rapolu, M.; Motoki, T.; Takeda, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. (2004). "Feedback Inhibition of Spinach L-Galactose Dehydrogenase by L-Ascorbate" (PDF). Plant and Cell Physiology 45 (9): 1271–1279. doi:10.1093/pcp/pch152. PMID 15509850.
- ↑ Grillet, L; Ouerdane L; Flis P; Hoang MTT; Isaure MP; Lobinski R; Curie C; Mari S (January 31, 2014). "Ascorbate Efflux as a New Strategy for Iron Reduction and Transport in Plants". The Journal of Biological Chemistry (289): 2515–2525. doi:10.1074/jbc.M113.514828.
- ↑ D-Erythroascorbic acid
- ↑ Eggersdorfer, M.; et al. (2005), "Vitamins", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a27_443
- ↑ China's grip on key food additive / The Christian Science Monitor. CSMonitor.com (2007-07-20). Retrieved on 2012-12-04.
- ↑ BASF’s description of vitamin C—developments in production methods. competition-commission.org.uk
- ↑ D vs. L ascorbate in natural products. kacst.edu.sa. Retrieved on 2012-12-04.
- ↑ Davies, Michael B.; Austin, John A.; Partridge, David A. (1991). Vitamin C : its chemistry and biochemistry. Cambridge [Cambridgeshire]: Royal Society of Chemistry. ISBN 9780851863337.
- ↑ "A Simple Test for Vitamin C" (PDF). School Science Review 83 (305): 131. 2002.
- ↑ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF). Retrieved 2010-02-04.
- ↑ "Japanese Pharmacopoeia, Fifteenth Edition" (PDF). 2006. Retrieved 2010-02-04.
Further reading
- Clayden; Greeves; Warren; Wothers (2001), Organic Chemistry, Oxford University Press, ISBN 0-19-850346-6.
- Davies, Michael B.; Austin, John; Partridge, David A., Vitamin C: Its Chemistry and Biochemistry, Royal Society of Chemistry, ISBN 0-85186-333-7.
- Coultate, T. P., Food: The Chemistry of Its Components (3rd ed.), Royal Society of Chemistry, ISBN 0-85404-513-9.
- Gruenwald, J.; Brendler, T.; Jaenicke, C., eds. (2004), PDR for Herbal Medicines (3rd ed.), Montvale, New Jersey: Thomson PDR.
- McMurry, John (2008), Organic Chemistry (7e ed.), Thomson Learning, ISBN 978-0-495-11628-8.
External links
- International Chemical Safety Card 0379
- SIDS Initial Assessment Report for L-Ascorbic acid from the Organisation for Economic Co-operation and Development (OECD)
- IPCS Poisons Information Monograph (PIM) 046
- Interactive 3D-structure of vitamin C with details on the x-ray structure
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||
|
