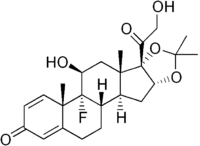
Triamcinolone acetonide
 | |
.png) | |
| Names | |
|---|---|
| IUPAC name
(4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-fluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one | |
| Identifiers | |
| 76-25-5 | |
| ChEBI | CHEBI:71418 |
| ChEMBL | ChEMBL1504 |
| ChemSpider | 6196 |
| 2867 | |
| Jmol interactive 3D | Image |
| PubChem | 6436 |
| UNII | F446C597KA |
| |
| |
| Properties | |
| C24H31FO6 | |
| Molar mass | 434.50 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Triamcinolone acetonide is a synthetic corticosteroid used to treat various skin conditions, to relieve the discomfort of mouth sores,[1] and in nasal spray form, to treat allergic rhinitis. It is a more potent derivative of triamcinolone, and is about eight times as potent as prednisone.[2]
It is also known under the brand names Kenalog (topical) and Volon A as an injection, to treat allergies, arthritis, eye diseases, intestinal problems, and skin diseases.[3] In 2014, the FDA made triamcinolone acetonide an over-the-counter drug in the USA in nasal spray form under the brand name Nasacort.[4]
Medical use
Triamcinolone acetonide as an intra-articular injectable has been used to treat a variety of musculoskeletal conditions. When applied as a topical ointment, it is used for blistering from poison ivy, oak, and sumac, applied to the skin, avoiding eyes, mouth, and genital area. It provides relatively immediate relief and is used before using oral prednisone. Oral and dental paste preparations are used for treating aphthous ulcers.
As an intravitreal injection, triamcinolone acetonide has been used to treat various eye diseases and has been found useful in reducing macular edema. Drug trials have found it to be as efficient as anti-VEGF drugs in eyes with artificial lenses over a two-year period.
Uncommonly, intramuscular injection of triamcinolone acetonide may be indicated for the control of severe or incapacitating allergic states for which conventional treatments have failed, such as asthma, atopic dermatitis, contact dermatitis, perennial or seasonal allergic rhinitis, serum sickness, and transfusion and drug hypersensitivity reactions.
Contraindications
As with all immunosuppressant drugs, triamcinolone acetonide should not be taken by persons suffering from infectious diseases, either currently or in recuperation or soon thereafter. Contact with infectious persons should be avoided during the entire course of treatment. Users who contract an infection during their regimen should contact their doctors to discuss whether to discontinue triamcinolone acetonide.
Triamcinolone acetonide should not be taken if the patient is also taking any other steroid or immunosuppressant, or if they have recently undergone any medical procedures involving the administration of steroids (e.g. nerve block).
Many drugs have been demonstrated to increase triamcinolone acetonide concentration in the blood to well above the intended level. Patients should inform doctors about any other drugs they are taking.
Triamcinolone acetonide should not be used by those with tuberculosis or untreated fungal, bacterial, systemic viral or herpes simplex infections without consulting a doctor first.
Veterinary use
Triamcinolone acetonide is also used in veterinary medicine as an ingredient in topical ointments and in topical sprays for control of pruritus in dogs.[5] A series of injections with triamcinolone acetonide or another corticosteroid may reduce keloid size and irritation. It is also used as a preinductor and/or inductor of birth in cows.
References
- ↑ Triamcinolone Acetonide Drug information from MedLine Plus
- ↑ Nasacort medication leaflet
- ↑ It's also important to discuss the possible risks of the injection. It has been know. To cause fat and muscle loss at injection site, leaving a large divot bone deep.
- ↑ Nasacort.com
- ↑ GENESIS® (triamcinolone acetonide) Topical Spray Drug information
| ||||||||||||||||||||||||||||||||||||||