Arimoclomol
 | |
| Systematic (IUPAC) name | |
|---|---|
|
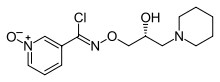
N-{[(2R)-2-hydroxy-3-piperidin-1-ylpropyl]oxy}pyridine-3-carboximidoyl chloride 1-oxide | |
| Clinical data | |
| Legal status |
|
| Routes of administration | Oral |
| Identifiers | |
| CAS Number |
289893-25-0 |
| ATC code | None |
| PubChem | CID 208924 |
| ChemSpider |
21106260 |
| UNII |
EUT3557RT5 |
| ChEMBL |
CHEMBL2107726 |
| Chemical data | |
| Formula | C14H20ClN3O3 |
| Molar mass | 313.78 g/mol |
| |
| |
| | |
Arimoclomol (INN; originally codenamed BRX-345, which is a citrate salt formulation of BRX-220) is an experimental drug developed by CytRx Corporation, a biopharmaceutical company based in Los Angeles, California. The orally administered drug is intended to treat amyotrophic lateral sclerosis (ALS).[1][2]
Mechanism of action
Arimoclomol is believed to function by stimulating a normal cellular protein repair pathway through the activation of molecular chaperones. Since damaged proteins, called aggregates, are thought to play a role in many diseases, CytRx believes that arimoclomol could treat a broad range of diseases.
Arimoclomol activates the heat shock response.[3][4][5][6][7][8] It is believed to act at Hsp70.[9]
History
Arimoclomol has been shown to extend life in an animal model of ALS[10] and was well tolerated in healthy human volunteers in a Phase I study. CytRx is currently conducting a Phase II clinical trial.[11]
Arimoclomol also has been shown to be an effective treatment in an animal model of Spinal Bulbar Muscular Atrophy (SBMA, also known as Kennedy's Disease). [12]
Arimoclomol was discovered by Hungarian researchers, as a drug candidate to treat insulin resistance[13][14] and diabetic complications such as retinopathy, neuropathy and nephropathy. Later, the compound, along with other small molecules, was screened for further development by Hungarian firm Biorex, which was sold to CytRx Corporation, who developed it toward a different direction from 2003.
References
- ↑ Cudkowicz ME, Shefner JM, Simpson E; et al. (July 2008). "Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis". Muscle Nerve 38 (1): 837–44. doi:10.1002/mus.21059. PMID 18551622.
- ↑ Traynor BJ, Bruijn L, Conwit R; et al. (July 2006). "Neuroprotective agents for clinical trials in ALS: a systematic assessment". Neurology 67 (1): 20–7. doi:10.1212/01.wnl.0000223353.34006.54. PMID 16832072.
- ↑ Kalmar B, Greensmith L (2009). "Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects". Cell. Mol. Biol. Lett. 14 (2): 319–35. doi:10.2478/s11658-009-0002-8. PMID 19183864.
- ↑ Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L (April 2004). "Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice". Nat. Med. 10 (4): 402–5. doi:10.1038/nm1021. PMID 15034571.
- ↑ Kalmar B, Greensmith L, Malcangio M, McMahon SB, Csermely P, Burnstock G (December 2003). "The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury". Exp. Neurol. 184 (2): 636–47. doi:10.1016/S0014-4886(03)00343-1. PMID 14769355.
- ↑ Rakonczay Z, Iványi B, Varga I; et al. (June 2002). "Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats". Free Radic. Biol. Med. 32 (12): 1283–92. doi:10.1016/S0891-5849(02)00833-X. PMID 12057766.
- ↑ Kalmar B, Burnstock G, Vrbová G, Urbanics R, Csermely P, Greensmith L (July 2002). "Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats". Exp. Neurol. 176 (1): 87–97. doi:10.1006/exnr.2002.7945. PMID 12093085.
- ↑ Benn SC, Brown RH (April 2004). "Putting the heat on ALS". Nat. Med. 10 (4): 345–7. doi:10.1038/nm0404-345. PMID 15057226.
- ↑ Brown IR (October 2007). "Heat shock proteins and protection of the nervous system". Ann. N. Y. Acad. Sci. 1113: 147–58. doi:10.1196/annals.1391.032. PMID 17656567.
- ↑ Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L (October 2008). "Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS". J. Neurochem. 107 (2): 339–50. doi:10.1111/j.1471-4159.2008.05595.x. PMID 18673445.
- ↑ "Phase II/III Randomized, Placebo-Controlled Trial of Arimoclomol in SOD1 Positive Familial Amyotrophic Lateral Sclerosis - Full Text View - ClinicalTrials.gov". Archived from the original on 11 May 2009. Retrieved 2009-05-18.
- ↑ Malik B, Nirmalananthan N, Gray A, La Spada A, Hanna M, Greensmith L (2013). "Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy". Brain 126 (3): 926–943. doi:10.1093/brain/aws343. PMID 23393146.
- ↑ Kürthy M, Mogyorósi T, Nagy K; et al. (June 2002). "Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models". Ann. N. Y. Acad. Sci. 967: 482–9. doi:10.1111/j.1749-6632.2002.tb04306.x. PMID 12079878.
- ↑ Seböková E, Kürthy M, Mogyorosi T; et al. (June 2002). "Comparison of the extrapancreatic action of BRX-220 and pioglitazone in the high-fat diet-induced insulin resistance". Ann. N. Y. Acad. Sci. 967: 424–30. doi:10.1111/j.1749-6632.2002.tb04298.x. PMID 12079870.
| ||||||||||||||||||