Arbutin
 | |
| Names | |
|---|---|
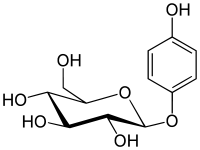
| IUPAC name
(2R,3S,4S,5R,6S)-2-Hydroxymethyl-6- (4-hydroxyphenoxy)oxane-3,4,5-triol | |
| Other names
Arbutoside Hydroquinone β-D-glucopyranoside | |
| Identifiers | |
| 497-76-7 | |
| ChEBI | CHEBI:18305 |
| ChEMBL | ChEMBL232202 |
| ChemSpider | 389765 |
| Jmol interactive 3D | Image |
| PubChem | 346 |
| RTECS number | CE8663000 |
| UNII | C5INA23HXF |
| |
| |
| Properties | |
| C12H16O7 | |
| Molar mass | 272.25 g·mol−1 |
| Appearance | colorless to white powder |
| Melting point | 199.5 °C (391.1 °F; 472.6 K) |
| Boiling point | 561.6 |
| 5.0 g/100 mL | |
| Solubility | soluble in alcohol slightly soluble in ethyl ether insoluble in benzene, chloroform, CS2 |
| log P | -1.35 |
| Vapor pressure | almost 0 (25 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| NFPA 704 | |
| Flash point | 293.4 °C (560.1 °F; 566.5 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Arbutin is a glycoside; a glycosylated hydroquinone extracted from the bearberry plant in the genus Arctostaphylos. It inhibits tyrosinase and thus prevents the formation of melanin. Arbutin is therefore used as a skin-lightening agent. Arbutin is found in wheat, and is concentrated in pear skins. It is also found in Bergenia crassifolia.[1] Arbutin was also produced by an in vitro culture of Schisandra chinensis.[2]
Production
Pure arbutin can be prepared synthetically from the reaction of acetobromoglucose and hydroquinone in the presence of alkali.[3]
Folk medicine
Bearberry, which contains arbutin, is a traditional treatment for urinary tract infections.[4]
Skin lightening agent
Bearberry extract is used in skin lightening treatments designed for long term and regular use. An active agent in brands of skin lightening preparations, it is more expensive than traditional skin lightening ingredients like hydroquinone, which is now banned in many countries. In vitro studies of human melanocytes exposed to arbutin at concentrations below 300 μg/mL reported decreased tyrosinase activity and melanin content with little evidence of cytotoxicity.[5]
Risks
Arbutin is glucosylated hydroquinone,[6] and may carry similar cancer risks,[7] although there are also claims that arbutin reduces cancer risk.[8] The German Institute of Food Research in Potsdam found that intestinal bacteria can transform arbutin into hydroquinone, which creates an environment favorable for intestinal cancer.[9]
References
- ↑ Carmen Pop, Laurian Vlase, Mircea Tamas (2009). "Natural Resources Containing Arbutin. Determination of Arbutin in the Leaves of Bergenia crassifolia (L.) Fritsch. acclimated in Romania". Not. Bot. Hort. Agrobot. Cluj 37 (1): 129–132.
- ↑ Dusková J, Dusek J, Jahodár L, Poustka F. (2005). "[Arbutin, salicin: the possibilities of their biotechnological production]". Ceska Slov Farm. 54 (2): 78–81.
- ↑ PubChem - Arbutin
- ↑ Garrett, J. T. (2003). The Cherokee Herbal: Native Plant Medicine from the Four Directions. Bear & Company. p. 209. ISBN 1879181967.
- ↑ Arbutin, Supporting Nomination for Toxicological Evaluation by the National Toxicology Program
- ↑ O'Donoghue, J L (September 2006). "Hydroquinone and its analogues in dermatology – a risk-benefit viewpoint". Journal of Cosmetic Dermatology 5 (3): 196–203. doi:10.1111/j.1473-2165.2006.00253.x. PMID 17177740.
The potential toxicity of HQ (hydroquinone) is dependent on the route of exposure
- ↑ Treatment of hyperpigmentation problems / skin lightening
- ↑ Bowman, Lee. July 25, 2005. Scripps Howard News Service. High yuck factor not necessarily good for us anymore
- ↑ Blaut M, Braune A, Wunderlich S, Sauer P, Schneider H, Glatt H (2006). "Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria". Food Chem. Toxicol. 44 (11): 1940–7. doi:10.1016/j.fct.2006.06.015. PMID 16904805.
