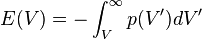Anton-Schmidt equation of state
The Anton-Schmidt equation is an empirical equation of state for crystalline solids, e.g. for pure metals or intermetallic compounds.[1] Quantum mechanical investigations of intermetallic compounds show that the dependency of the pressure under isotropic deformation can be described empirically by
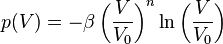 .
.
Integration of 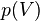 leads to equation of the state for the total energy. The energy
leads to equation of the state for the total energy. The energy 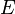 required to compress a solid to volume
required to compress a solid to volume 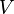 is
is
which gives
![E(V) = \frac{\beta V_0}{n+1} \left(\frac{V}{V_0}\right)^{n+1} \left[\ln\left(\frac{V}{V_0}\right) - \frac{1}{n+1}\right] - E_\infty](../I/m/9dd49b35511ff7af2a2d1a4edcf58d4d.png) .
.
The fitting parameters 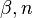 and
and 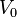 are related to material properties, where
are related to material properties, where
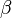 is the bulk modulus
is the bulk modulus  at equilibrium volume
at equilibrium volume 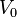 .
. correlates with the Grüneisen parameter
correlates with the Grüneisen parameter 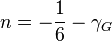 .[2][3]
.[2][3]
However, the fitting parameter  does not reproduce the total energy of the free atoms.[4]
does not reproduce the total energy of the free atoms.[4]
The total energy equation is used to determine elastic and thermal material constants in quantum chemical simulation packages.[4][5]
See also
References
- ↑ Mayer, B.; Anton, H.; Bott, E.; Methfessel, M.; Sticht, J.; Harris, J.; Schmidt, P.C. (2003). "Ab-initio calculation of the elastic constants and thermal expansion coefficients of Laves phases". Intermetallics 11 (1): 23–32. doi:10.1016/S0966-9795(02)00127-9. ISSN 0966-9795.
- ↑ Otero-de-la-Roza, et al., Gibbs2: A new version of the quasi-harmonic model code. Computer Physics Communications 182.8 (2011): 1708-1720. DOI: 10.1016/j.cpc.2011.04.016
- ↑ Jund, Philippe, et al., Physical properties of thermoelectric zinc antimonide using first-principles calculations., Physical Review B 85.22 (2012) .
- ↑ 4.0 4.1 Atomic Simulation Environment documentation of the Technical University of Denmark, Department of Physics
- ↑ Gilgamesh chemical software documentation of the Department of Chemical Engineering of Carnegie Mellon University
This article is issued from Wikipedia - version of the Monday, June 08, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.