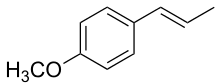
Anethole
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(E)-1-Methoxy-4-(1-propenyl)benzene | |
| Other names
para-mMethoxyphenylpropene p-Propenylanisole Isoestragole trans-1-Methoxy-4-(prop-1-enyl)benzene | |
| Identifiers | |
| 104-46-1 | |
| ChEBI | CHEBI:35616 |
| ChEMBL | ChEMBL452630 |
| ChemSpider | 553166 |
| Jmol interactive 3D | Image |
| KEGG | D02377 |
| PubChem | 637563 |
| UNII | 21C2F5E8RE |
| |
| |
| Properties | |
| C10H12O | |
| Molar mass | 148.21 g·mol−1 |
| Density | 0.998 g/cm3 |
| Melting point | 20 to 21 °C (68 to 70 °F; 293 to 294 K) |
| Boiling point | 234 °C (453 °F; 507 K) (81 °C at 2 mmHg) |
| Hazards | |
| Safety data sheet | External MSDS |
| Related compounds | |
| Related compounds |
Anisole; Estragole |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Anethole (anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils. It contributes a large component of the odor and flavor of anise and fennel (both in the botanical family Apiaceae), anise myrtle (Myrtaceae), liquorice (Fabaceae), camphor, magnolia blossoms, and star anise (Illiciaceae). Closely related to anethole is its isomer estragole, abundant in tarragon (Asteraceae) and basil (Lamiaceae), that has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid.[1] Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This difference causes certain anise-flavored liqueurs to become opaque when diluted with water, the ouzo effect.
Structure and production
Anethole is an aromatic, unsaturated ether related to lignols. It exists as both cis-trans isomers (see also E-Z notation), involving the double bond outside the ring. The more abundant isomer, and the one preferred for use, is the trans or E isomer.
Like related compounds, anethole is poorly soluble in water. Historically, this property was used to detect adulteration in samples.[2]
Most anethole is obtained from terpentine-like extracts from trees.[1][3] Also of only minor commercial significance, anethole can be isolated from essential oils.[4][5]
| Essential oil | World production | trans-anethole |
|---|---|---|
| Anise | 8 tons (1999) | 95% |
| Star anise | 400 tons (1999), mostly from China | 87% |
| Fennel | 25 tons (1999), mostly from Spain | 70% |
It is also readily prepared from anisole and propionic acid via the intermediacy of 4-methoxypropiophenone.[1]
Uses
Flavoring
It is distinctly sweet, measuring 13 times sweeter than sugar. It is perceived as being pleasant to the taste even at higher concentrations. It is used in alcoholic drinks ouzo , ((rakı)) and Pernod. It is also used in seasoning and confectionery applications, oral hygiene products, and in small quantities in natural berry flavors.[5]
Precursor to other compounds
Because they metabolize anethole into several aromatic chemical compounds, some bacteria are candidates for use in commercial bioconversion of anethole to more valuable materials.[6] Bacterial strains capable of using trans-anethole as the sole carbon source include JYR-1 (Pseudomonas putida)[7] and TA13 (Arthrobacter aurescens).[6]
Research
Antimicrobial and antifungal activity
Anethole has potent antimicrobial properties, against bacteria, yeast, and fungi.[8] Reported antibacterial properties include both bacteriostatic and bactericidal action against Salmonella enterica[9] but not when used against Salmonella via a fumigation method.[10] Antifungal activity includes increasing the effectiveness of some other phytochemicals (e.g. polygodial) against Saccharomyces cerevisiae and Candida albicans;[11] In vitro, anethole has antihelmintic action on eggs and larvae of the sheep gastrointestinal nematode Haemonchus contortus.[12] Anethole also has nematicidal activity against the plant nematode Meloidogyne javanica in vitro and in pots of cucumber seedlings.[13]
Insecticidal activity
Anethole also is a promising insecticide. Several essential oils consisting mostly of anethole have insecticidal action against larvae of the mosquitos Ochlerotatus caspius[14] and Aedes aegypti.[15][16] In similar manner, anethole itself is effective against the fungus gnat Lycoriella ingenua (Sciaridae)[17] and the mold mite Tyrophagus putrescentiae.[18] Against the mite, anethole is a slightly more effective pesticide than DEET, but anisaldehyde, a related natural compound that occurs with anethole in many essential oils, is 14 times more effective.[18] The insecticidal action of anethole is greater as a fumigant than as a contact agent. (E)-anethole is highly effective as a fumigant against the cockroach Blattella germanica[19] and against adults of the weevils Sitophilus oryzae, Callosobruchus chinensis and beetle Lasioderma serricorne.[20]
As well as an insect pesticide, anethole is an effective insect repellent against mosquitos.[21]

Anethole is responsible for the "ouzo effect", the spontaneous formation of a microemulsion[22][23] that gives many alcoholic beverages containing anethole and water their cloudy appearance. Such a spontaneous microemulsion has many potential commercial applications in the food and pharmaceutical industries.[24]
Precursor to illicit drugs
Anethole is an inexpensive chemical precursor for paramethoxyamphetamine (PMA),[25] and is used in its clandestine manufacture.[26] Anethole is present in the essential oil from guarana, which is alleged to have a psychoactive effect. The absence of PMA or any other known psychoactive derivative of anethole in human urine after ingestion of guarana leads to the conclusion that any purported psychoactive effect of guarana is not due to aminated anethole metabolites.[27] Anethole is also present in absinthe, a liquor with a reputation for psychoactive effects; these effects however are attributed to ethanol.[28] (See also thujone, anethole dithione (ADT), and anethole trithione (ATT)).
Safety
Formerly generally recognized as safe (GRAS), after a hiatus anethole was reaffirmed by Flavor and Extract Manufacturers Association (FEMA) as GRAS.[29] The hiatus was due to concerns about liver toxicity and possible carcinogenic activity, reported in rats.[30] Anethole is associated with a slight increase in liver cancer in rats,[30] although the evidence is scant and generally regarded as evidence that anethole is not a carcinogen.[30][31] An evaluation of anethole by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) found its notable pharmacologic properties to be reduction in motor activity, lowering of body temperature, and hypnotic, analgesic, and anticonvulsant effects.[32] A subsequent evaluation by JECFA found some reason for concern regarding carcinogenicity, but there is currently insufficient data to support this.[33] At this time, the JECFA summary of these evaluations is that anethole has no safety concern at current levels of intake when used as a flavoring agent.[34]
In large quantities, anethole is slightly toxic and may act as an irritant.[35]
See also
- Category:Anise liqueurs and spirits
- List of liqueurs#Anise-flavored liqueurs
- Chavicol
- Safrole
- Fenchone
References
- 1 2 3 Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim: 2002. Published online: 15 January 2003; doi:10.1002/14356007.a11_141.
- ↑ S. Waldbott (1920). "Essential oils". Chemical Abstracts 14 (17): 3753–3755.
- ↑ Davis, Curry B. (February 20, 1990). "United States Patent 4902850: Purification of anethole by crystallization". Free Patents Online.
- ↑ Ram Nath Chopra and I. C. Chopra and K. L. Handa and L. D. Kapur (1958). Chopra's Indigenous Drugs of India (2nd ed.). Academic Publishers. pp. 178–179. ISBN 978-81-85086-80-4.
- 1 2 Philip R. Ashurst (1999). Food Flavorings. Springer. p. 460. ISBN 978-0-8342-1621-1.
- 1 2 Shimoni E, Baasov T, Ravid U, Shoham Y (2002). "The trans-anethole degradation pathway in an Arthrobacter sp". J. Biol. Chem. 277 (14): 11866–72. doi:10.1074/jbc.M109593200. PMID 11805095.
- ↑ Ryu J, Seo J, Lee Y, Lim Y, Ahn JH, Hur HG (July 2005). "Identification of syn- and anti-anethole-2,3-epoxides in the metabolism of trans-anethole by the newly isolated bacterium Pseudomonas putida JYR-1". J. Agric. Food Chem. 53 (15): 5954–8. doi:10.1021/jf040445x. PMID 16028980.
- ↑ De M, De AK, Sen P, Banerjee AB (February 2002). "Antimicrobial properties of star anise (Illicium verum Hook f)". Phytother Res 16 (1): 94–5. doi:10.1002/ptr.989. PMID 11807977.
- ↑ Kubo I, Fujita K (December 2001). "Naturally occurring anti-Salmonella agents". J. Agric. Food Chem. 49 (12): 5750–4. doi:10.1021/jf010728e. PMID 11743758.
- ↑ Weissinger WR, McWatters KH, Beuchat LR (April 2001). "Evaluation of volatile chemical treatments for lethality to Salmonella on alfalfa seeds and sprouts". J. Food Prot. 64 (4): 442–50. PMID 11307877.
- ↑ Fujita K, Fujita T, Kubo I (January 2007). "Anethole, a potential antimicrobial synergist, converts a fungistatic dodecanol to a fungicidal agent". Phytother Res 21 (1): 47–51. doi:10.1002/ptr.2016. PMID 17078111.
- ↑ Camurça-Vasconcelos AL, Bevilaqua CM, Morais SM, Maciel MV, Costa CT, Macedo IT, Oliveira LM, Braga RR, Silva RA, Vieira LS (2007). "Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils". Vet. Parasitol. 148 (3–4): 288–94. doi:10.1016/j.vetpar.2007.06.012. PMID 17629623.
- ↑ Oka Y, Nacar S, Putievsky E, Ravid U, Yaniv Z, Spiegel Y (July 2000). "Nematicidal activity of essential oils and their components against the root-knot nematode". Phytopathology 90 (7): 710–5. doi:10.1094/PHYTO.2000.90.7.710. PMID 18944489.
- ↑ Knio KM, Usta J, Dagher S, Zournajian H, Kreydiyyeh S (2008). "Larvicidal activity of essential oils extracted from commonly used herbs in Lebanon against the seaside mosquito, Ochlerotatus caspius". Bioresour. Technol. 99 (4): 763–8. doi:10.1016/j.biortech.2007.01.026. PMID 17368893.
- ↑ Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST (July 2004). "Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances". J. Agric. Food Chem. 52 (14): 4395–400. doi:10.1021/jf0497152. PMID 15237942.
- ↑ Morais SM, Cavalcanti ES, Bertini LM, Oliveira CL, Rodrigues JR, Cardoso JH (2006). "Larvicidal activity of essential oils from Brazilian Croton species against Aedes aegypti L". J. Am. Mosq. Control Assoc. 22 (1): 161–4. doi:10.2987/8756-971X(2006)22[161:LAOEOF]2.0.CO;2. PMID 16646345.
- ↑ Park IK, Choi KS, Kim DH, Choi IH, Kim LS, Bak WC, Choi JW, Shin SC (August 2006). "Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae)". Pest Manag. Sci. 62 (8): 723–8. doi:10.1002/ps.1228. PMID 16786497.
- 1 2 Lee HS (June 2005). "Food protective effect of acaricidal components isolated from anise seeds against the stored food mite, Tyrophagus putrescentiae (Schrank)". J. Food Prot. 68 (6): 1208–10. PMID 15954709.
- ↑ Chang KS, Ahn YJ (February 2002). "Fumigant activity of (E)-anethole identified in Illicium verum fruit against Blattella germanica". Pest Manag. Sci. 58 (2): 161–6. doi:10.1002/ps.435. PMID 11852640.
- ↑ Kim DH, Ahn YJ (March 2001). "Contact and fumigant activities of constituents of Foeniculum vulgare fruit against three coleopteran stored-product insects". Pest Manag. Sci. 57 (3): 301–6. doi:10.1002/ps.274. PMID 11455661.
- ↑ Padilha de Paula J, Gomes-Carneiro MR, Paumgartten FJ (2003). "Chemical composition, toxicity and mosquito repellency of Ocimum selloi oil". J Ethnopharmacol 88 (2–3): 253–60. doi:10.1016/s0378-8741(03)00233-2. PMID 12963152.
- ↑ Sitnikova, Natalia L.; Rudolf Sprik, Gerard Wegdam and Erika Eiser (2005). "Spontaneously Formed trans-Anethol/Water/Alcohol Emulsions: Mechanism of Formation and Stability" (PDF). Langmuir 21 (16): 7083–7089. doi:10.1021/la046816l. PMID 16042427. Archived (PDF) from the original on 18 March 2009. Retrieved 2009-03-15.
- ↑ David Carteau, Dario Bassani, and Isabelle Pianet (April–May 2008). "The "Ouzo effect": Following the spontaneous emulsification of trans-anethole in water by NMR". Comptes Rendus Chimie 11 (4–5): 493–498. doi:10.1016/j.crci.2007.11.003.
- ↑ Spernath A, Aserin A (2006). "Microemulsions as carriers for drugs and nutraceuticals". Adv Colloid Interface Sci. 128–130: 47–64. doi:10.1016/j.cis.2006.11.016. PMID 17229398.
- ↑ Waumans D, Bruneel N, Tytgat J (2003). "Anise oil as para-methoxyamphetamine (PMA) precursor". Forensic Sci. Int. 133 (1–2): 159–70. doi:10.1016/S0379-0738(03)00063-X. PMID 12742705.
- ↑ Waumans D, Hermans B, Bruneel N, Tytgat J (2004). "A neolignan-type impurity arising from the peracid oxidation reaction of anethole in the surreptitious synthesis of 4-methoxyamphetamine (PMA)". Forensic Sci. Int. 143 (2–3): 133–9. doi:10.1016/j.forsciint.2004.02.033. PMID 15240033.
- ↑ Benoni H, Dallakian P, Taraz K (1996). "Studies on the essential oil from guarana". Z Lebensm Unters Forsch 203 (1): 95–8. doi:10.1007/BF01267777. PMID 8765992.
- ↑ Lachenmeier DW (March 2008). "[Thujone-attributable effects of absinthe are only an urban legend—toxicology uncovers alcohol as real cause of absinthism]". Med Monatsschr Pharm (in German) 31 (3): 101–6. PMID 18429531.
- ↑ Newberne P, Smith RL, Doull J, Goodman JI, Munro IC, Portoghese PS, Wagner BM, Weil CS, Woods LA, Adams TB, Lucas CD, Ford RA (1999). "The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Flavour and Extract Manufacturer's Association". Food Chem. Toxicol. 37 (7): 789–811. doi:10.1016/S0278-6915(99)00037-X. PMID 10496381.
- 1 2 3 Newberne PM, Carlton WW, Brown WR (1989). "Histopathological evaluation of proliferative liver lesions in rats fed trans-anethole in chronic studies". Food Chem. Toxicol. 27 (1): 21–6. doi:10.1016/0278-6915(89)90087-2. PMID 2467866.
- ↑ Waddell WJ (2002). "Thresholds of carcinogenicity of flavors". Toxicol. Sci. 68 (2): 275–9. doi:10.1093/toxsci/68.2.275. PMID 12151622.
- ↑ "Trans-anethole". WHO Food Additives Series 14 (466). International Program on Chemical Safety (IPCS).
- ↑ "Trans-anethole". WHO Food Additives Series 28 (717). International Program on Chemical Safety (IPCS). 1998.
- ↑ "Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives: Trans-anethole". International Program on Chemical Safety (IPCS). November 12, 2001. Archived from the original on 11 March 2009. Retrieved March 10, 2009.
- ↑ "Safety data for anethole". Physical & Theoretical Chemistry Laboratory Safety, Oxford University. Retrieved March 10, 2009.
| ||||||