Anemia
| Anemia | |
|---|---|
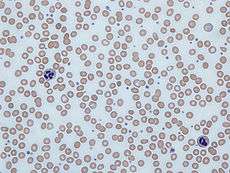 Human blood from a case of iron-deficiency anemia | |
| Classification and external resources | |
| Pronunciation | /əˈniːmiə/ |
| Specialty | Hematology |
| ICD-10 | D50-D64 |
| ICD-9-CM | 280-285 |
| DiseasesDB | 663 |
| MedlinePlus | 000560 |
| eMedicine | med/132 emerg/808 emerg/734 |
| MeSH | D000740 |
Anemia, also spelt anaemia, is usually defined as a decrease in the amount of red blood cells (RBCs) or hemoglobin in the blood.[1][2] It can also be defined as a lowered ability of the blood to carry oxygen.[3] When anemia comes on slowly the symptoms are often vague and may include: feeling tired, weakness, shortness of breath or a poor ability to exercise. Anemia that comes on quickly often has greater symptoms which may include: confusion, feeling like one is going to pass out, loss of consciousness, or increased thirst. Anemia must be significant before a person becomes noticeably pale. Additional symptoms may occur depending on the underlying cause.[4]
There are three main types of anemia: that due to blood loss, that due to decreased red blood cell production, and that due to increased red blood cell breakdown. Causes of blood loss include trauma and gastrointestinal bleeding, among others. Causes of decreased production include iron deficiency, a lack of vitamin B12, thalassemia and a number of neoplasms of the bone marrow among others. Causes of increased breakdown include a number of genetic conditions such as sickle cell anemia, infections like malaria and some autoimmune diseases among others. It can also be classified based on the size of red blood cells and amount of hemoglobin in each cell. If the cells are small it is microcytic anemia, if they are large it is macrocytic anemia and if they are normal sized it is normocytic anemia. Diagnosis in men is based on a hemoglobin of less than 130 to 140 g/L (13 to 14 g/dL), while in women it must be less than 120 to 130 g/L (12 to 13 g/dL).[4][5] Further testing is then required to determine the cause.[4]
Certain groups of individuals, such as pregnant women, benefit from the use of iron pills for prevention.[4][6] Dietary supplementation, without determining the specific cause, is not recommended. The use of blood transfusions is typically based on a person's signs and symptoms.[4] In those without symptoms they are not recommended unless hemoglobin levels are less than 60 to 80 g/L (6 to 8 g/dL).[4][7] These recommendations may also apply to some people with acute bleeding.[4] Erythropoiesis-stimulating medications are only recommended in those with severe anemia.[7]
Anemia is the most common disorder of the blood with it affecting about a quarter of people globally.[4] Iron-deficiency anemia affects nearly 1 billion.[8] In 2013 anemia due to iron deficiency resulted in about 183,000 deaths – down from 213,000 deaths in 1990.[9] It is more common in females than males,[8] among children, during pregnancy, and in the elderly.[4] Anemia increases costs of medical care and lowers a person's productivity through a decreased ability to work.[5] The name is derived from Ancient Greek: ἀναιμία anaimia, meaning "lack of blood", from ἀν- an-, "not" + αἷμα haima, "blood".[10]
Signs and symptoms
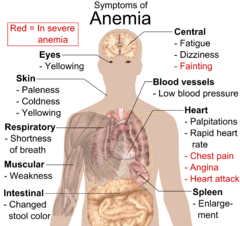
Anemia goes undetected in many people, and symptoms can be minor or vague. The symptoms can be related to the underlying cause or the anemia itself. Most commonly, people with anemia report feelings of weakness, or fatigue, general malaise, and sometimes poor concentration. They may also report dyspnea (shortness of breath) on exertion. In very severe anemia, the body may compensate for the lack of oxygen-carrying capability of the blood by increasing cardiac output. The patient may have symptoms related to this, such as palpitations, angina (if pre-existing heart disease is present), intermittent claudication of the legs, and symptoms of heart failure. On examination, the signs exhibited may include pallor (pale skin, lining mucosa, conjunctiva and nail beds), but this is not a reliable sign. There may be signs of specific causes of anemia, e.g., koilonychia (in iron deficiency), jaundice (when anemia results from abnormal break down of red blood cells — in hemolytic anemia), bone deformities (found in thalassemia major) or leg ulcers (seen in sickle-cell disease). In severe anemia, there may be signs of a hyperdynamic circulation: tachycardia (a fast heart rate), bounding pulse, flow murmurs, and cardiac ventricular hypertrophy (enlargement). There may be signs of heart failure. Pica, the consumption of non-food items such as ice, but also paper, wax, or grass, and even hair or dirt, may be a symptom of iron deficiency, although it occurs often in those who have normal levels of hemoglobin. Chronic anemia may result in behavioral disturbances in children as a direct result of impaired neurological development in infants, and reduced scholastic performance in children of school age. Restless legs syndrome is more common in those with iron-deficiency anemia.
Causes
The causes of anemia may be classified as impaired red blood cell (RBC) production, increased RBC destruction (hemolytic anemias), blood loss and fluid overload (hypervolemia). Several of these may interplay to cause anemia eventually. Indeed, the most common cause of anemia is blood loss, but this usually does not cause any lasting symptoms unless a relatively impaired RBC production develops, in turn most commonly by iron deficiency.[13] (See Iron deficiency anemia)
Impaired production
- Disturbance of proliferation and differentiation of stem cells
- Pure red cell aplasia[14]
- Aplastic anemia[14] affects all kinds of blood cells. Fanconi anemia is a hereditary disorder or defect featuring aplastic anemia and various other abnormalities.
- Anemia of renal failure[14] by insufficient erythropoietin production
- Anemia of endocrine disorders
- Disturbance of proliferation and maturation of erythroblasts
- Pernicious anemia[14] is a form of megaloblastic anemia due to vitamin B12 deficiency dependent on impaired absorption of vitamin B12. Lack of dietary B12 causes non-pernicious megaloblastic anemia
- Anemia of folic acid deficiency,[14] as with vitamin B12, causes megaloblastic anemia
- Anemia of prematurity, by diminished erythropoietin response to declining hematocrit levels, combined with blood loss from laboratory testing, generally occurs in premature infants at two to six weeks of age.
- Iron deficiency anemia, resulting in deficient heme synthesis[14]
- Thalassemias, causing deficient globin synthesis[14]
- Congenital dyserythropoietic anemias, causing ineffective erythropoiesis
- Anemia of renal failure[14] (also causing stem cell dysfunction)
- Other mechanisms of impaired RBC production
- Myelophthisic anemia[14] or myelophthisis is a severe type of anemia resulting from the replacement of bone marrow by other materials, such as malignant tumors or granulomas.
- Myelodysplastic syndrome[14]
- anemia of chronic inflammation[14]
Increased destruction
Anemias of increased red blood cell destruction are generally classified as hemolytic anemias. These are generally featuring jaundice and elevated lactate dehydrogenase levels.
- Intrinsic (intracorpuscular) abnormalities[14] cause premature destruction. All of these, except paroxysmal nocturnal hemoglobinuria, are hereditary genetic disorders.[15]
- Hereditary spherocytosis[14] is a hereditary defect that results in defects in the RBC cell membrane, causing the erythrocytes to be sequestered and destroyed by the spleen.
- Hereditary elliptocytosis[14] is another defect in membrane skeleton proteins.
- Abetalipoproteinemia,[14] causing defects in membrane lipids
- Enzyme deficiencies
- Pyruvate kinase and hexokinase deficiencies,[14] causing defect glycolysis
- Glucose-6-phosphate dehydrogenase deficiency and glutathione synthetase deficiency,[14] causing increased oxidative stress
- Hemoglobinopathies
- Sickle cell anemia[14]
- Hemoglobinopathies causing unstable hemoglobins[14]
- Paroxysmal nocturnal hemoglobinuria[14]
- Extrinsic (extracorpuscular) abnormalities
- Antibody-mediated
- Warm autoimmune hemolytic anemia is caused by autoimmune attack against red blood cells, primarily by IgG. It is the most common of the autoimmune hemolytic diseases.[16] It can be idiopathic, that is, without any known cause, drug-associated or secondary to another disease such as systemic lupus erythematosus, or a malignancy, such as chronic lymphocytic leukemia.[17][17]
- Cold agglutinin hemolytic anemia is primarily mediated by IgM. It can be idiopathic[18] or result from an underlying condition.
- Rh disease,[14] one of the causes of hemolytic disease of the newborn
- Transfusion reaction to blood transfusions[14]
- Mechanical trauma to red cells
- Microangiopathic hemolytic anemias, including thrombotic thrombocytopenic purpura and disseminated intravascular coagulation[14]
- Infections, including malaria[14]
- Heart surgery
- Haemodialysis
- Antibody-mediated
Blood loss
- Anemia of prematurity from frequent blood sampling for laboratory testing, combined with insufficient RBC production
- Trauma[14] or surgery, causing acute blood loss
- Gastrointestinal tract lesions,[14] causing either acute bleeds (e.g. variceal lesions, peptic ulcers or chronic blood loss (e.g. angiodysplasia)
- Gynecologic disturbances,[14] also generally causing chronic blood loss
- From menstruation, mostly among young women or older women who have fibroids
- Infection by intestinal nematodes feeding on blood, such as hookworms[19] and the whipworm Trichuris trichiura.[20]
Fluid overload
Fluid overload (hypervolemia) causes decreased hemoglobin concentration and apparent anemia:
- General causes of hypervolemia include excessive sodium or fluid intake, sodium or water retention and fluid shift into the intravascular space.[21]
- Anemia of pregnancy is induced by blood volume expansion experienced in pregnancy.
Diagnosis

Anemia is typically diagnosed on a complete blood count. Apart from reporting the number of red blood cells and the hemoglobin level, the automatic counters also measure the size of the red blood cells by flow cytometry, which is an important tool in distinguishing between the causes of anemia. Examination of a stained blood smear using a microscope can also be helpful, and it is sometimes a necessity in regions of the world where automated analysis is less accessible.
In modern counters, four parameters (RBC count, hemoglobin concentration, MCV and RDW) are measured, allowing others (hematocrit, MCH and MCHC) to be calculated, and compared to values adjusted for age and sex. Some counters estimate hematocrit from direct measurements.
| Age or gender group | Hb threshold (g/dl) | Hb threshold (mmol/l) |
|---|---|---|
| Children (0.5–5.0 yrs) | 11.0 | 6.8 |
| Children (5–12 yrs) | 11.5 | 7.1 |
| Teens (12–15 yrs) | 12.0 | 7.4 |
| Women, non-pregnant (>15yrs) | 12.0 | 7.4 |
| Women, pregnant | 11.0 | 6.8 |
| Men (>15yrs) | 13.0 | 8.1 |
Reticulocyte counts, and the "kinetic" approach to anemia, have become more common than in the past in the large medical centers of the United States and some other wealthy nations, in part because some automatic counters now have the capacity to include reticulocyte counts. A reticulocyte count is a quantitative measure of the bone marrow's production of new red blood cells. The reticulocyte production index is a calculation of the ratio between the level of anemia and the extent to which the reticulocyte count has risen in response. If the degree of anemia is significant, even a "normal" reticulocyte count actually may reflect an inadequate response. If an automated count is not available, a reticulocyte count can be done manually following special staining of the blood film. In manual examination, activity of the bone marrow can also be gauged qualitatively by subtle changes in the numbers and the morphology of young RBCs by examination under a microscope. Newly formed RBCs are usually slightly larger than older RBCs and show polychromasia. Even where the source of blood loss is obvious, evaluation of erythropoiesis can help assess whether the bone marrow will be able to compensate for the loss, and at what rate. When the cause is not obvious, clinicians use other tests, such as: ESR, ferritin, serum iron, transferrin, RBC folate level, serum vitamin B12, hemoglobin electrophoresis, renal function tests (e.g. serum creatinine) although the tests will depend on the clinical hypothesis that is being investigated. When the diagnosis remains difficult, a bone marrow examination allows direct examination of the precursors to red cells, although is rarely used as is painful, invasive and is hence reserved for cases where severe pathology needs to be determined or excluded.
Red blood cell size
In the morphological approach, anemia is classified by the size of red blood cells; this is either done automatically or on microscopic examination of a peripheral blood smear. The size is reflected in the mean corpuscular volume (MCV). If the cells are smaller than normal (under 80 fl), the anemia is said to be microcytic; if they are normal size (80–100 fl), normocytic; and if they are larger than normal (over 100 fl), the anemia is classified as macrocytic. This scheme quickly exposes some of the most common causes of anemia; for instance, a microcytic anemia is often the result of iron deficiency. In clinical workup, the MCV will be one of the first pieces of information available, so even among clinicians who consider the "kinetic" approach more useful philosophically, morphology will remain an important element of classification and diagnosis. Limitations of MCV include cases where the underlying cause is due to a combination of factors - such as iron deficiency (a cause of microcytosis) and vitamin B12 deficiency (a cause of macrocytosis) where the net result can be normocytic cells.
Production vs. destruction or loss
The "kinetic" approach to anemia yields arguably the most clinically relevant classification of anemia. This classification depends on evaluation of several hematological parameters, particularly the blood reticulocyte (precursor of mature RBCs) count. This then yields the classification of defects by decreased RBC production versus increased RBC destruction and/or loss. Clinical signs of loss or destruction include abnormal peripheral blood smear with signs of hemolysis; elevated LDH suggesting cell destruction; or clinical signs of bleeding, such as guaiac-positive stool, radiographic findings, or frank bleeding. The following is a simplified schematic of this approach:
* For instance, sickle cell anemia with superimposed iron deficiency; chronic gastric bleeding with B12 and folate deficiency; and other instances of anemia with more than one cause.
** Confirm by repeating reticulocyte count: ongoing combination of low reticulocyte production index, normal MCV and hemolysis or loss may be seen in bone marrow failure or anemia of chronic disease, with superimposed or related hemolysis or blood loss.
Here is a schematic representation of how to consider anemia with MCV as the starting point:
| Anemia | |||||||||||||||||||||||||||||||||||||||||||||
| Macrocytic anemia (MCV>100) | Normocytic anemia (MCV 80–100) | Microcytic anemia (MCV<80) | |||||||||||||||||||||||||||||||||||||||||||
| High reticulocyte count | Low reticulocyte count | ||||||||||||||||||||||||||||||||||||||||||||
Other characteristics visible on the peripheral smear may provide valuable clues about a more specific diagnosis; for example, abnormal white blood cells may point to a cause in the bone marrow.
Microcytic
Microcytic anemia is primarily a result of hemoglobin synthesis failure/insufficiency, which could be caused by several etiologies:
- Heme synthesis defect
- Iron deficiency anemia (microcytosis is not always present)
- Anemia of chronic disease (more commonly presenting as normocytic anemia)
- Globin synthesis defect
- Alpha-, and beta-thalassemia
- HbE syndrome
- HbC syndrome
- Various other unstable hemoglobin diseases
- Sideroblastic defect
- Hereditary sideroblastic anemia
- Acquired sideroblastic anemia, including lead toxicity
- Reversible sideroblastic anemia
Iron deficiency anemia is the most common type of anemia overall and it has many causes. RBCs often appear hypochromic (paler than usual) and microcytic (smaller than usual) when viewed with a microscope.
- Iron deficiency anemia is due to insufficient dietary intake or absorption of iron to meet the body's needs. Infants, toddlers, and pregnant women have higher than average needs. Increased iron intake is also needed to offset blood losses due to digestive tract issues, frequent blood donations, or heavy menstrual periods.[23] Iron is an essential part of hemoglobin, and low iron levels result in decreased incorporation of hemoglobin into red blood cells. In the United States, 12% of all women of childbearing age have iron deficiency, compared with only 2% of adult men. The incidence is as high as 20% among African American and Mexican American women.[24] Studies have shown iron deficiency without anemia causes poor school performance and lower IQ in teenage girls, although this may be due to socioeconomic factors.[25][26] Iron deficiency is the most prevalent deficiency state on a worldwide basis. It is sometimes the cause of abnormal fissuring of the angular (corner) sections of the lips (angular stomatitis).
- In the United States, the most common cause of iron deficiency is bleeding or blood loss, usually from the gastrointestinal tract. Fecal occult blood testing, upper endoscopy and lower endoscopy should be performed to identify bleeding lesions. In older men and women, the chances are higher that bleeding from the gastrointestinal tract could be due to colon polyps or colorectal cancer.
- Worldwide, the most common cause of iron deficiency anemia is parasitic infestation (hookworms, amebiasis, schistosomiasis and whipworms).[27]
The Mentzer index (mean cell volume divided by the RBC count) predicts whether microcytic anemia may be due to iron deficiency or thallasemia, although it requires confirmation.
Macrocytic
- Megaloblastic anemia, the most common cause of macrocytic anemia, is due to a deficiency of either vitamin B12, folic acid, or both. Deficiency in folate and/or vitamin B12 can be due either to inadequate intake or insufficient absorption. Folate deficiency normally does not produce neurological symptoms, while B12 deficiency does.
- Pernicious anemia is caused by a lack of intrinsic factor, which is required to absorb vitamin B12 from food. A lack of intrinsic factor may arise from an autoimmune condition targeting the parietal cells (atrophic gastritis) that produce intrinsic factor or against intrinsic factor itself. These lead to poor absorption of vitamin B12.
- Macrocytic anemia can also be caused by removal of the functional portion of the stomach, such as during gastric bypass surgery, leading to reduced vitamin B12/folate absorption. Therefore, one must always be aware of anemia following this procedure.
- Hypothyroidism
- Alcoholism commonly causes a macrocytosis, although not specifically anemia. Other types of liver disease can also cause macrocytosis.
- Drugs such as Methotrexate, zidovudine, and other substances may inhibit DNA replication such as heavy metals (e.g. Lead)
Macrocytic anemia can be further divided into "megaloblastic anemia" or "nonmegaloblastic macrocytic anemia". The cause of megaloblastic anemia is primarily a failure of DNA synthesis with preserved RNA synthesis, which results in restricted cell division of the progenitor cells. The megaloblastic anemias often present with neutrophil hypersegmentation (six to 10 lobes). The nonmegaloblastic macrocytic anemias have different etiologies (i.e. unimpaired DNA globin synthesis,) which occur, for example, in alcoholism. In addition to the nonspecific symptoms of anemia, specific features of vitamin B12 deficiency include peripheral neuropathy and subacute combined degeneration of the cord with resulting balance difficulties from posterior column spinal cord pathology.[28] Other features may include a smooth, red tongue and glossitis. The treatment for vitamin B12-deficient anemia was first devised by William Murphy, who bled dogs to make them anemic, and then fed them various substances to see what (if anything) would make them healthy again. He discovered that ingesting large amounts of liver seemed to cure the disease. George Minot and George Whipple then set about to isolate the curative substance chemically and ultimately were able to isolate the vitamin B12 from the liver. All three shared the 1934 Nobel Prize in Medicine.[29]
Normocytic
Normocytic anemia occurs when the overall hemoglobin levels are decreased, but the red blood cell size (mean corpuscular volume) remains normal. Causes include:
- Acute blood loss
- Anemia of chronic disease
- Aplastic anemia (bone marrow failure)
- Hemolytic anemia
Dimorphic
A dimorphic appearance on a peripheral blood smear occurs when there are two simultaneous populations of red blood cells, typically of different size and hemoglobin content (this last feature affecting the color of the red blood cell on a stained peripheral blood smear). For example, a person recently transfused for iron deficiency would have small, pale, iron deficient red blood cells (RBCs) and the donor RBCs of normal size and color. Similarly, a person transfused for severe folate or vitamin B12 deficiency would have two cell populations, but, in this case, the patient's RBCs would be larger and paler than the donor's RBCs. A person with sideroblastic anemia (a defect in heme synthesis, commonly caused by alcoholism, but also drugs/toxins, nutritional deficiencies, a few acquired and rare congenital diseases) can have a dimorphic smear from the sideroblastic anemia alone. Evidence for multiple causes appears with an elevated RBC distribution width (RDW), indicating a wider-than-normal range of red cell sizes, also seen in common nutritional anemia.
Heinz body anemia
Heinz bodies form in the cytoplasm of RBCs and appear as small dark dots under the microscope. Heinz body anemia has many causes, and some forms can be drug-induced. It is triggered in cats by eating onions[30] or acetaminophen (paracetamol). It can be triggered in dogs by ingesting onions or zinc, and in horses by ingesting dry red maple leaves.
Hyperanemia
Hyperanemia is a severe form of anemia, in which the hematocrit is below 10%.
Refractory anemia
Refractory anemia, an anemia which does not respond to treatment,[31] is often seen secondary to myelodysplastic syndromes.[32] Iron deficiency anemia may also be refractory as a clinical manifestation of gastrointestinal problems which disrupt iron absorption or cause occult bleeding. [33]
Treatments
Treatments for anemia depend on cause and severity. Vitamin supplements given orally (folic acid or vitamin B12) or intramuscularly (vitamin B12) will replace specific deficiencies.
Oral iron
Nutritional iron deficiency is common in developing nations. An estimated two-thirds of children and of women of childbearing age in most developing nations are estimated to suffer from iron deficiency; one-third of them have the more severe form of the disorder, anemia.[34] Iron deficiency from nutritional causes is rare in men and postmenopausal women. The diagnosis of iron deficiency mandates a search for potential sources of loss, such as gastrointestinal bleeding from ulcers or colon cancer. Mild to moderate iron-deficiency anemia is treated by oral iron supplementation with ferrous sulfate, ferrous fumarate, or ferrous gluconate. When taking iron supplements, stomach upset and/or darkening of the feces are commonly experienced. The stomach upset can be alleviated by taking the iron with food; however, this decreases the amount of iron absorbed. Vitamin C aids in the body's ability to absorb iron, so taking oral iron supplements with orange juice is of benefit. In anemias of chronic disease, associated with chemotherapy, or associated with renal disease, some clinicians prescribe recombinant erythropoietin or epoetin alfa, to stimulate RBC production, although since there is also concurrent iron deficiency and inflammation present, parenteral iron is advised to be taken concurrently.[35]
Injectable iron
In cases where oral iron has either proven ineffective, would be too slow (for example, pre-operatively) or where absorption is impeded (for example in cases of inflammation), parenteral iron can be used. The body can absorb up to 6 mg iron daily from the gastrointestinal tract. In many cases the patient has a deficit of over 1,000 mg of iron which would require several months to replace. This can be given concurrently with erythropoietin to ensure sufficient iron for increased rates of erythropoiesis.
Blood transfusions
Blood transfusions in those without symptoms is not recommended until the hemoglobin is below 60 to 80 g/L (6 to 8 g/dL).[4] In those with coronary artery disease who are not actively bleeding transfusions are only recommended when the hemoglobin is below 70 to 80g/L (7 to 8 g/dL).[7] Transfusing earlier does not improve survival.[36] Transfusions otherwise should only be undertaken in cases of cardiovascular instability.[37]
Erythropoiesis-stimulating agent
The motive for the administration of an erythropoiesis-stimulating agent (ESA) is to maintain hemoglobin at the lowest level that both minimizes transfusions and meets the individual persons needs.[38] They should not be used for mild or moderate anemia.[36] They are not recommended in people with chronic kidney disease unless hemoglobin levels are less than 10 g/dL or they have symptoms of anemia. Their use should be along with parenteral iron.[38][39]
Hyperbaric oxygen
Treatment of exceptional blood loss (anemia) is recognized as an indication for hyperbaric oxygen (HBO) by the Undersea and Hyperbaric Medical Society.[40][41] The use of HBO is indicated when oxygen delivery to tissue is not sufficient in patients who cannot be given blood transfusions for medical or religious reasons. HBO may be used for medical reasons when threat of blood product incompatibility or concern for transmissible disease are factors.[40] The beliefs of some religions (ex: Jehovah's Witnesses) may require they use the HBO method.[40] A 2005 review of the use of HBO in severe anemia found all publications reported positive results.[42]
Epidemiology
A moderate degree of iron-deficiency anemia affected approximately 610 million people worldwide or 8.8% of the population.[8] It is slightly more common in females (9.9%) than males (7.8%).[8] Mild iron deficiency anemia affects another 375 million.[8]
History
Evidence of anemia goes back more than 4000 years.[43]
References
- ↑ "What Is Anemia? - NHLBI, NIH". www.nhlbi.nih.gov. Retrieved 2016-01-31.
- ↑ Stedman's medical dictionary (28th ed.). Philadelphia: Lippincott Williams & Wilkins. 2006. p. Anemia. ISBN 9780781733908.
- ↑ Rodak, Bernadette F. (2007). Hematology : clinical principles and applications (3rd ed.). Philadelphia: Saunders. p. 220. ISBN 9781416030065.
- 1 2 3 4 5 6 7 8 9 10 Janz, TG; Johnson, RL; Rubenstein, SD (Nov 2013). "Anemia in the emergency department: evaluation and treatment.". Emergency medicine practice 15 (11): 1–15; quiz 15–6. PMID 24716235.
- 1 2 Smith RE, Jr (Mar 2010). "The clinical and economic burden of anemia.". The American journal of managed care. 16 Suppl Issues: S59–66. PMID 20297873.
- ↑ Bhutta, ZA; Das, JK; Rizvi, A; Gaffey, MF; Walker, N; Horton, S; Webb, P; Lartey, A; Black, RE; Lancet Nutrition Interventions Review, Group; Maternal and Child Nutrition Study, Group (Aug 3, 2013). "Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?". Lancet 382 (9890): 452–77. doi:10.1016/S0140-6736(13)60996-4. PMID 23746776.
- 1 2 3 Qaseem, A; Humphrey, LL; Fitterman, N; Starkey, M; Shekelle, P; Clinical Guidelines Committee of the American College of, Physicians (Dec 3, 2013). "Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians.". Annals of internal medicine 159 (11): 770–9. doi:10.7326/0003-4819-159-11-201312030-00009. PMID 24297193.
- 1 2 3 4 5 Vos, T; Flaxman, AD; Naghavi, M; Lozano, R; Michaud, C; Ezzati, M; Shibuya, K; Salomon, JA; et al. (Dec 15, 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet 380 (9859): 2163–96. doi:10.1016/S0140-6736(12)61729-2. PMID 23245607.
- ↑ GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013.". Lancet 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ↑ "anaemia". Dictionary.com. Retrieved 7 July 2014.
- ↑ eMedicineHealth > anemia article Author: Saimak T. Nabili, MD, MPH. Editor: Melissa Conrad Stöppler, MD. Last Editorial Review: 12/9/2008. Retrieved on 4 April 2009
- ↑ Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda; Usa, 20894. "Red Blood Cells - National Library of Medicine". PubMed Health. Retrieved 2016-01-31.
- ↑ National Heart Lung and Blood Institute > What Causes Anemia? Retrieved on June 9, 2010
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Table 12-1 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson. Robbins Basic Pathology. Philadelphia: Saunders. ISBN 1-4160-2973-7. 8th edition.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; & Mitchell, Richard N. (2007). Robbins Basic Pathology (8th ed.). Saunders Elsevier. p. 432 ISBN 978-1-4160-2973-1
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease. St. Louis, Mo: Elsevier Saunders. p. 637. ISBN 0-7216-0187-1.
- 1 2 AUTOIMMUNE HEMOLYTIC ANEMIA (AIHA) By J.L. Jenkins. The Regional Cancer Center. 2001 Archived October 7, 2009 at the Wayback Machine
- ↑ Berentsen S, Beiske K, Tjønnfjord GE (October 2007). "Primary chronic cold agglutinin disease: An update on pathogenesis, clinical features and therapy". Hematology 12 (5): 361–70. doi:10.1080/10245330701445392. PMC 2409172. PMID 17891600.
- ↑ Brooker S; Hotez PJ; Bundy DA (2008). "Hookworm-related anaemia among pregnant women: a systematic review". PLoS Neglected Tropical Diseases 2 (9): e291. doi:10.1371/journal.pntd.0000291. PMC 2553481. PMID 18820740.
- ↑ Gyorkos TW; Gilbert NL; Larocque R; Casapía M (2011). "Trichuris and hookworm infections associated with anaemia during pregnancy". Tropical Medicine & International Health 16 (4): 531–7. doi:10.1111/j.1365-3156.2011.02727.x.
- ↑ Page 62 (Fluid imbalances) in: Portable Fluids and Electrolytes (Portable Series). Hagerstwon, MD: Lippincott Williams & Wilkins. 2007. ISBN 1-58255-678-4.
- ↑ World Health Organization (2008). Worldwide prevalence of anaemia 1993–2005 (PDF). Geneva: World Health Organization. ISBN 978-92-4-159665-7. Archived (PDF) from the original on 12 March 2009. Retrieved 2009-03-25.
- ↑ Recommendations to Prevent and Control Iron Deficiency in the United States MMWR 1998;47 (No. RR-3) p. 5
- ↑ "Iron Deficiency --- United States, 1999--2000". MMWR 51 (40): 897–899. October 11, 2002. Retrieved 21 April 2012.
- ↑ Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG (2001). "Iron Deficiency and Cognitive Achievement Among School-Aged Children and Adolescents in the United States". Pediatrics 107 (6): 1381–1386. doi:10.1542/peds.107.6.1381. PMID 11389261.
- ↑ Grantham-McGregor S, Ani C (2001). "Iron-Deficiency Anemia: Reexamining the Nature and Magnitude of the Public Health Problem". J Nutr 131 (2): 649S–668S. PMID 11160596.
- ↑ "Iron Deficiency Anaemia: Assessment, Prevention, and Control: A guide for programme managers" (PDF). Retrieved 2010-08-24.
- ↑ eMedicine – Vitamin B-12 Associated Neurological Diseases : Article by Niranjan N Singh, MD, DM, DNB July 18, 2006
- ↑ "Physiology or Medicine 1934 – Presentation Speech". Nobelprize.org. 1934-12-10. Archived from the original on 28 August 2010. Retrieved 2010-08-24.
- ↑ "Onions are Toxic to Cats". Peteducation.com. Archived from the original on 3 September 2010. Retrieved 2010-08-24.
- ↑ "MedTerms Definition: Refractory Anemia". Medterms.com. 2011-04-27. Retrieved 2011-10-31.
- ↑ "Good Source for later". Atlasgeneticsoncology.org. Retrieved 2011-10-31.
- ↑ Mody RJ, Brown PI, Wechsler DS; Brown; Wechsler (February 2003). "Refractory iron deficiency anemia as the primary clinical manifestation of celiac disease". J. Pediatr. Hematol. Oncol. 25 (2): 169–72. doi:10.1097/00043426-200302000-00018. PMID 12571473.
- ↑ West CE (November 1996). "Strategies to control nutritional anemia". Am. J. Clin. Nutr. 64 (5): 789–90. PMID 8901803.
- ↑ http://guidance.nice.org.uk/CG114/Guidance/pdf/English
- 1 2 Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D; Dyer; Englander; Fu; Freeman; Kagen (Dec 3, 2013). "Treatment of anemia in patients with heart disease: a systematic review". Annals of internal medicine 159 (11): 746–57. doi:10.7326/0003-4819-159-11-201312030-00007. PMID 24297191.
- ↑ Goddard AF, James MW, McIntyre AS, Scott BB; James; McIntyre; Scott; British Society of Gastroenterology (2011). "Guidelines for the management of iron deficiency anaemia". Gut 60 (10): 1309–1316. doi:10.1136/gut.2010.228874. PMID 21561874.
- 1 2 Aapro MS, Link H; Link (2008). "September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents". Oncologist. 13 Suppl 3 (Supplement 3): 33–6. doi:10.1634/theoncologist.13-S3-33. PMID 18458123.
- ↑ American Society of Nephrology, "Five Things Physicians and Patients Should Question" (PDF), Choosing Wisely: an initiative of the ABIM Foundation (American Society of Nephrology), retrieved August 17, 2012
- 1 2 3 Undersea and Hyperbaric Medical Society. "Exceptional Blood Loss — Anemia". Archived from the original on July 5, 2008. Retrieved 2008-05-19.
- ↑ Hart GB, Lennon PA, Strauss MB. (1987). "Hyperbaric oxygen in exceptional acute blood-loss anemia". J. Hyperbaric Med 2 (4): 205–210. Retrieved 2008-05-19.
- ↑ Van Meter KW (2005). "A systematic review of the application of hyperbaric oxygen in the treatment of severe anemia: an evidence-based approach". Undersea Hyperb Med 32 (1): 61–83. PMID 15796315. Retrieved 2008-05-19.
- ↑ Tayles, N (Sep 1996). "Anemia, genetic diseases, and malaria in prehistoric mainland Southeast Asia". American Journal of Physical Anthropology 101 (1): 11–27. doi:10.1002/(SICI)1096-8644(199609)101:1<11::AID-AJPA2>3.0.CO;2-G. PMID 8876811.
External links
- National Anemia Action Council (USA)
- Anemia - Lab Tests Online
| ||||||||||||||||||||||||||||||||||||||||||||||||
|