Metal ammine complex
-3D-balls.png)
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.[1]
History
Ammine complexes played a major role in the development of coordination chemistry, specifically determination of the stereochemistry and structure. They are easily prepared, and the metal-nitrogen ratio can be determined by elemental analysis. One of the first ammine complexes to be described was "Magnus' green salt" [Pt(NH3)4][PtCl4].[2] Relying mainly on the ammine complexes, Alfred Werner developed his Nobel Prize-winning concept of the structure of coordination compounds (see Figure).[3][4]
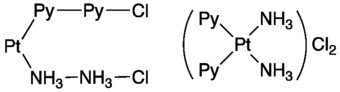
Examples
Cobalt(III) and chromium(III)
In the history of coordination chemistry, the ammines of chromium(III) and cobalt(III) were of significance. Both families of amines are relatively inert kinetically, which allows the separation of isomers.[6] For example, tetraamminedichlorochromium(III) chloride, [Cr(NH3)4Cl2]Cl, has two forms - the cis isomer is violet, while the trans isomer is green. The trichloride of the hexaammine (hexamminecobalt(III) chloride, [Co(NH3)6]Cl3)) exists as only a single isomer. "Reinecke's salt" with the formula NH4[Cr(NCS)4(NH3)2].H2O was first reported in 1863.[7]
Zinc(II) and Nickel(II) and Copper(II) and silver(I)

Zinc(II) forms tetraamminezinc with the formula [Zn(NH3)4]2+.[8] like almost all zinc complexes, it is colourless. The hexaammines of nickel and copper are violet and deep blue ammine, respectively. The latter is characteristic of the presence of copper(II) in qualitative inorganic analysis.
The diamminesilver ion ([Ag(NH3)2]+) is an example of a linear diammine complex.[9] It is this complex that forms when otherwise rather insoluble silver chloride dissolves in aqueous ammonia. The compound ([Ag(NH3)2]NO3 is the active ingredient in Tollen's reagent.
Reactions
Ligand exchange and redox reactions
Since ammonia is a stronger ligand in the spectrochemical series than water, metal ammine complexes are stabilized relative to the corresponding aquo complexes. For similar reasons, metal ammine complexes are less strongly oxidizing than are the corresponding aquo complexes. The latter property is illustrated by the stability of [Co(NH3)6]3+ in aqueous solution and the nonexistence of [Co(H2O)6]3+ (which oxidizes water).
Acid-base reactions
Once complexed to a metal ion, ammonia is not basic. This property is illustrated by the stability of some metal ammine complexes in strong acid solutions. When the M-NH3 bond is weak, the ammine ligand dissociates and protonation ensues. The behavior is illustrated by the non-reaction and reaction with [Co(NH3)6]3+ and [Ni(NH3)6]2+, respectively.
The ammine ligands are more acidic than is ammonia (pKa ~ 33). For highly cationic complexes such as [Pt(NH3)6]4+, the conjugate base can be obtained. The deprotonation of cobalt(III) ammine-halide complexes, e.g. [CoCl(NH3)5]2+ labilises the Co-Cl bond, according to the Sn1CB mechanism.
Applications
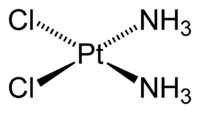
Metal ammine complexes find many uses. Cisplatin (PtCl2(NH3)2) is a coordination compound containing two chloro and two ammine ligands. This a drug used in treating cancer.[10] Many other amine complexes of the platinum group metals have been evaluated for this application.
In the separation of the individual platinum metals from their ore, several schemes rely on the precipitation of [RhCl(NH3)5]Cl2. In some separation schemes, palladium is purified by manipulating equilibria involving [Pd(NH3)4]Cl2, PdCl2(NH3)2, and Pt(NH3)4[PdCl4].
In the processing of cellulose, the copper ammine complex known as Schweizer's reagent ([Cu(NH3)4(H2O)2](OH)2) is sometimes used to solubilise the polymer. Schweizer's reagent is prepared by treating an aqueous solutions of copper(II) ions with ammonia. Initially, the light blue hydroxide precipitates only to redissolve upon addition of more ammonia:
- [Cu(H2O)6]2+ + 2 OH− → Cu(OH)2 + 6 H2O
- Cu(OH)2 + 4 NH3 + 2 H2O → [Cu(NH3)4(H2O)2]2+ + 2 OH−
See also
References
- ↑ A. von Zelewsky "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995. ISBN 0-471-95599-X.
- ↑ Atoji, M.; Richardson, J. W.; Rundle, R. E. (1957). "On the Crystal Structures of the Magnus Salts, Pt(NH3)4PtCl4". J. Am. Chem. Soc. 79 (12): 3017–3020. doi:10.1021/ja01569a009.
- ↑ "Werner Centennial" George B. Kauffman, Ed. Adv. Chem. Ser., 1967, Volume 62. ISBN 978-0-8412-0063-0
- ↑ von Zelewsky, A. "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995. ISBN 0-471-95599-X.
- ↑ Alfred Werner "Beitrag zur Konstitution anorganischer Verbindungen" Zeitschrift für anorganische Chemie 1893, Volume 3, pages 267–330.doi:10.1002/zaac.18930030136
- ↑ Basolo, F.; Pearson, R. G. "Mechanisms of Inorganic Reactions." John Wiley and Son: New York: 1967. ISBN 0-471-05545-X
- ↑ Reinecke, A. "Über Rhodanchromammonium-Verbindungen" Annalen der Chemie und Pharmacie, volume 126, pages 113-118 (1863). doi: 10.1002/jlac.18631260116.
- ↑ Zinc hydroxide in excess ammonia (with pictures)
- ↑ Nilsson, K. B., Persson, I., Kessler, V. G., "Coordination Chemistry of the Solvated AgI and AuI Ions in Liquid and Aqueous Ammonia, Trialkyl and Triphenyl Phosphite, and Tri-n-butylphosphine Solutions", Inorganic Chemistry 2006, volume 45, pp. 6912. doi:10.1021/ic060175v
- ↑ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.