Amidine
Amidines are a class of oxoacid derivatives.
The oxoacid from which an amidine is derived must be of the form RnE(=O)OH, where R is a substituent. The −OH group is replaced by an −NH2 group and the =O group is replaced by =NR, giving amidines the general structure RnE(=NR)NR2.[1][2][3]
Carboxamidines

When the parent oxoacid is a carboxylic acid, the resulting amidine is a carboxamidine or carboximidamide (IUPAC name), and has the following general structure:

Carboxamidines are frequently referred to simply as amidines, as they are the most commonly encountered type of amidine in organic chemistry. The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines include DBU, diminazene, and benzamidine.
The most common way to make primary amidines is by the Pinner reaction.
Properties
Amidines are much more basic than amides and are among the strongest neutral bases.[4]
Protonation occurs onto the sp² hybridized nitrogen. This occurs because the positive charge can be delocalized onto both nitrogen atoms:
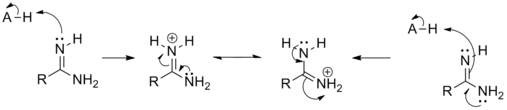
The amidinium cation has identical C-N bond lengths.
Amidinate salts
An amidinate salt has the general structure M+[RNRCNR]− [5] and can be accessed by reaction of a carbodiimide with an organometallic compound such as methyl lithium. They are used widely as ligands in organometallic complexes.
See also
- Guanidines - a similar group of compounds where the central Carbon is bonded to three Nitrogens.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amidines".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carboxamidines".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "sulfinamidines".
- ↑ Clayden; Greeves; Warren (2001). Organic chemistry. Oxford university press. p. 202. ISBN 978-0-19-850346-0.
- ↑ Chemistry and Technology of Carbodiimides Henri Ulrich