Amantadine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
Adamantan-1-amine | |
| Clinical data | |
| Trade names | Symmetrel |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682064 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 86–90%[1] |
| Protein binding | 67%[1] |
| Metabolism | Minimal (mostly to acetyl metabolites)[1] |
| Biological half-life | 10–31 hours[1] |
| Excretion | Urine[1] |
| Identifiers | |
| CAS Number |
768-94-5 |
| ATC code | N04BB01 |
| PubChem | CID 2130 |
| IUPHAR/BPS | 4128 |
| DrugBank |
DB00915 |
| ChemSpider |
2045 |
| UNII |
BF4C9Z1J53 |
| KEGG |
D07441 |
| ChEBI |
CHEBI:2618 |
| ChEMBL |
CHEMBL660 |
| Synonyms | 1-Adamantylamine |
| Chemical data | |
| Formula | C10H17N |
| Molar mass | 151.249 g/mol |
| |
| |
| (verify) | |
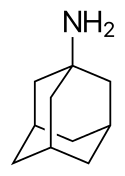
Amantadine (trade name Symmetrel, by Endo Pharmaceuticals) is a drug that has U.S. Food and Drug Administration approval for use both as an antiviral and an antiparkinsonian drug. It is the organic compound 1-adamantylamine or 1-aminoadamantane, meaning it consists of an adamantane backbone that has an amino group substituted at one of the four methyne positions. Rimantadine is a closely related derivative of adamantane with similar biological properties.
Apart from medical uses, this compound is useful as a building block, allowing the insertion of an adamantyl group.
According to the U.S. Centers for Disease Control and Prevention, 100% of seasonal H3N2 and 2009 pandemic flu samples tested have shown resistance to adamantanes, and amantadine is no longer recommended for treatment of influenza in the United States. Additionally, its effectiveness as an antiparkinsonian drug is undetermined, with a 2003 Cochrane Review concluding that there was insufficient evidence in support of or against its efficacy and safety.[2]
Medical uses
Parkinson's disease
Amantadine is a weak antagonist of the NMDA-type glutamate receptor, increases dopamine release, and blocks dopamine reuptake.[3] This makes it a weak therapy for Parkinson's disease. Although, as an antiparkinsonian, it can be used as monotherapy, or together with L-DOPA to treat L-DOPA-related motor fluctuations (i.e., shortening of L-DOPA duration of clinical effect, probably related to progressive neuronal loss) and L-DOPA-related dyskinesias (choreiform movements associated with long-term L-DOPA use, probably related to chronic pulsatile stimulation of dopamine receptors).
A 2003 Cochrane review of the scientific literature concluded evidence was inadequate to support the use of amantadine for Parkinson's disease.[2]
Influenza
Amantadine is no longer recommended for treatment of influenza A infection. For the 2008/2009 flu season, the United States' Centers for Disease Control and Prevention (CDC) found that 100% of seasonal H3N2 and 2009 pandemic flu samples tested have shown resistance to adamantanes.[4] The CDC issued an alert to doctors to prescribe the neuraminidase inhibitors oseltamivir and zanamivir instead of amantadine and rimantadine for treatment of flu.[5][6] A 2014 Cochrane review did not find benefit for the prevention or treatment of influenza A.[7]
Fatigue in multiple sclerosis
Amantadine also seems to have moderate effects on multiple sclerosis (MS) related fatigue.[8]
Adverse effects
Amantadine has been associated with several central nervous system (CNS) side effects, likely due to amantadine's dopaminergic and adrenergic activity, and to a lesser extent, its activity as an anticholinergic. CNS side effects include nervousness, anxiety, agitation, insomnia, difficulty in concentrating, and exacerbations of pre-existing seizure disorders and psychiatric symptoms in patients with schizophrenia or Parkinson's disease. The usefulness of amantadine as an anti-parkinsonian drug is somewhat limited by the need to screen patients for a history of seizures and psychiatric symptoms.
Rare cases of severe skin rashes, such as Stevens-Johnson syndrome,[9] and of suicidal ideation have also been reported in patients treated with amantadine.[10][11]
Livedo reticularis is a possible side effect of amantadine use for Parkinson's disease.[12]
Mechanism of action
Influenza
The mechanisms for amantadine's antiviral and antiparkinsonian effects are unrelated. The mechanism of amantadine's antiviral activity involves interference with the viral protein, M2, a proton channel.[13][14] After entry of the virus into cells via endocytosis, it is localized in acidic vacuoles; the M2 channel functions in transporting protons with the gradient from the vacuolar space into to interior of the virion. Acidification of the interior results in disassociation of ribonucleoproteins, and the onset of viral replication. Amantadine and rimantadine function in a mechanistically identical fashion in entering the barrel of the tetrameric M2 channel, and blocking pore function (i.e., proton translocation). Resistance to the drug class is a consequence of mutations to the pore-lining residues of the channel, leading to the inability of the sterically bulky adamantane ring that both share in entering in their usual way, into the channel.
Influenza B strains possess a structurally distinct M2 channels with channel-facing side chains that fully obstruct the channel vis-a-vis binding of adamantine-class channel inhibitors, while still allowing proton flow and channel function to occur; this constriction in the channels is responsible for the ineffectiveness of this drug and rimantadine towards all circulating Influenza B strains.
Parkinson's disease
Amantadine appears to act through several pharmacological mechanisms, but no dominant mechanism of action has been identified. It is a dopaminergic, noradrenergic and serotonergic substance, blocks NMDA receptors, and seems to raise beta-endorphin/beta-lipotropin levels. Amantadine probably does not inhibit MAO enzyme.[15] Moreover, the mechanism of its antiparkinsonian effect is poorly understood. The drug has many effects in the brain, including release of dopamine and norepinephrine from nerve endings. It appears to be a weak NMDA receptor antagonist[16][17] as well as an anticholinergic, specifically a nicotinic alpha-7 antagonist like the similar pharmaceutical memantine.
History
Amantadine was approved by the U.S. Food and Drug Administration in October 1966 as a prophylactic agent against Asian influenza, and eventually received approval for the treatment of influenzavirus A[18][19][20][21] in adults. In 1969, the drug was also discovered by accident to help reduce symptoms of Parkinson's disease, drug-induced extrapyramidal syndromes, and akathisia.
Research
In a 2012 study, 184 patients with severe traumatic brain injury were treated with amantadine or placebo for four weeks. In this study, the drug accelerated functional brain recovery during treatment. However, the placebo group had improved just as much as the amantadine group at six weeks — two weeks after the drug administration ended.[22]
Veterinary misuse
In 2005, Chinese poultry farmers were reported to have used amantadine to protect birds against avian influenza.[23] In Western countries and according to international livestock regulations, amantadine is approved only for use in humans. Chickens in China have received an estimated 2.6 billion doses of amantadine.[23] Avian flu (H5N1) strains in China and southeast Asia are now resistant to amantadine, although strains circulating elsewhere still seem to be sensitive. If amantadine-resistant strains of the virus spread, the drugs of choice in an avian flu outbreak will probably be restricted to the scarcer and costlier oseltamivir and zanamivir, which work by a different mechanism and are less likely to trigger resistance.
September 23, 2015 — The U.S. Food and Drug Administration has announced the recall of Dingo Chip Twists “Chicken in the Middle” dog treats because it has the potential to be contaminated with amantadine. [24]
See also
References
- 1 2 3 4 5 "SYMMETREL® (amantadine hydrochloride)" (PDF). TGA eBusiness Services. NOVARTIS Pharmaceuticals Australia Pty Limited. 29 June 2011. Retrieved 24 February 2014.
- 1 2 Crosby, Niall J; Deane, Katherine; Clarke, Carl E (2003). Clarke, Carl E, ed. "Amantadine in Parkinson's disease". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003468.
- ↑ Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. p. 3962. ISBN 978-3-85200-181-4.
- ↑ CDC weekly influenza report - week 35, cdc.gov
- ↑ "CDC Recommends against the Use of Amantadine and Rimantadine for the Treatment or Prophylaxis of Influenza in the United States during the 2005–06 Influenza Season". CDC Health Alert. Centers for Disease Control and Prevention. 2006-01-14. Archived from the original on 3 May 2008. Retrieved 2008-05-20.
- ↑ Deyde, Varough M.; Xu, Xiyan; Bright, Rick A.; Shaw, Michael; Smith, Catherine B.; Zhang, Ye; Shu, Yuelong; Gubareva, Larisa V.; Cox, Nancy J.; Klimov, Alexander I. (2007). "Surveillance of Resistance to Adamantanes among Influenza A(H3N2) and A(H1N1) Viruses Isolated Worldwide". Journal of Infectious Diseases 196 (2): 249–257. doi:10.1086/518936. PMID 17570112.
- ↑ Alves Galvão, MG; Rocha Crispino Santos, MA; Alves da Cunha, AJ (21 November 2014). "Amantadine and rimantadine for influenza A in children and the elderly.". The Cochrane database of systematic reviews 11: CD002745. doi:10.1002/14651858.CD002745.pub4. PMID 25415374.
- ↑ "Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment.". Sleep 33 (8): 1061–7. Aug 2010. PMID 20815187.
- ↑ K C Singhal & S Z Rahman, Stevens Johnson Syndrome induced by Amantadine, Rational Drug Bulletin, 2002, Vol. 12, No. 1: 6
- ↑ Endo Pharmaceuticals (May 2003). "Symmetrel (Amantadine) Prescribing Information" (PDF). Retrieved 2007-08-02.
- ↑ Cook, PE; Dermer, SW; McGurk, T (1986). "Fatal overdose with amantadine". Canadian Journal of Psychiatry 31 (8): 757–8. PMID 3791133.
- ↑ Vollum DI, Parkes JD, Doyle D (June 1971). "Livedo reticularis during amantadine treatment". Br Med J 2 (5762): 627–8. doi:10.1136/bmj.2.5762.627. PMC 1796527. PMID 5580722.
- ↑ Wang C, Takeuchi K, Pinto LH, Lamb RA (1993). "Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block". Journal of Virology 67 (9): 5585–94. PMC 237962. PMID 7688826.
- ↑ Jing X, Ma C, Ohigashi Y; et al. (2008). "Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel". Proc. Natl. Acad. Sci. U.S.A. 105 (31): 10967–72. doi:10.1073/pnas.0804958105. PMC 2492755. PMID 18669647.
- ↑ http://onlinelibrary.wiley.com/doi/10.1111/j.1600-0773.1971.tb00646.x/abstract
- ↑ Kornhuber J, Bormann J, Hübers M, Rusche K, Riederer P (1991) "Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: a human postmortem brain study." Eur.J.Pharmacol.Mol.Pharmacol.Sect. 206:297-300.
- ↑ Blanpied TA, Clarke RJ, Johnson JW (2005). "Amantadine inhibits NMDA receptors by accelerating channel closure during channel block". Journal of Neuroscience 25 (13): 3312–22. doi:10.1523/JNEUROSCI.4262-04.2005. PMID 15800186.
- ↑ David A. Hounshell and John Kenly Smith, "Science and Corporate Strategy: Du Pont R&D, 1902-1980", 1988, Cambridge University Press, p. 469.
- ↑ "Sales of flu drug by du Pont unit a 'disappointment'" (Last accessed May 19, 2008.) October 5, 1982, The New York Times.
- ↑ Maugh, T. (1979). "Panel urges wide use of antiviral drug". Science 206 (4422): 1058–60. doi:10.1126/science.386515. PMID 386515.
- ↑ Maugh, T. H. (1976). "Amantadine: an Alternative for Prevention of Influenza". Science 192 (4235): 130–1. doi:10.1126/science.192.4235.130. PMID 17792438.
- ↑ Giacino, J. T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D. I.; Cho, S.; Yablon, S. A.; Luther, M.; Hammond, F. M.; Nordenbo, A.; Novak, P.; Mercer, W.; Maurer-Karattup, P.; Sherer, M. (2012). "Placebo-Controlled Trial of Amantadine for Severe Traumatic Brain Injury". New England Journal of Medicine 366 (9): 819–826. doi:10.1056/NEJMoa1102609. PMID 22375973.
- 1 2 Sipress, Alan (2005-06-18). "Bird Flu Drug Rendered Useless". Washington Post. pp. A01. Retrieved 2007-08-02.
- ↑
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||