Amagat
An amagat is a practical unit of number density. Although it can be applied to any substance at any conditions, it is defined as the number of ideal gas molecules per unit volume at 1 atm (= 101.325 kPa) and 0 °C (= 273.15 K).[1] It is named after Émile Amagat, who also has Amagat's law named after him. The abbreviated form of amagat is "amg."
Definition
Number density in amg, denoted here by  , is defined as
, is defined as
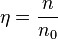 ,
,
where n0 = 1 amg = 2.686 7805×1025 m−3 = 44.615 036 mol/m3 is the Loschmidt constant.
In practice, number density of an ideal gas at pressure P and temperature T can be calculated as[2]
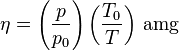 ,
,
where T0 = 273.15 K and p0 = 101.325 kPa.
Example
Number density of an ideal gas (such as air) at room temperature (20 °C) and 1 atm (101.325 kPa) is
 .
.
References
- ↑ Hirschfelder, Joseph O.; Curtiss, Charles F.; Bird, R. Byron (1967), Molecular Theory of Gases and Liquids (Corrected printing ed.), John Wiley & Sons, Inc.
- ↑ In this formula, absolute units of pressure and temperature, relative to vacuum and absolute zero, must be used.
This article is issued from Wikipedia - version of the Friday, August 21, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.